|
Water–gas Shift Reaction
The water-gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen: : CO + H2O CO2 + H2 The water gas shift reaction was discovered by Italian physicist Felice Fontana in 1780. It was not until much later that the industrial value of this reaction was realized. Before the early 20th century, hydrogen was obtained by reacting steam under high pressure with iron to produce iron oxide and hydrogen. With the development of industrial processes that required hydrogen, such as the Haber–Bosch ammonia synthesis, a less expensive and more efficient method of hydrogen production was needed. As a resolution to this problem, the WGSR was combined with the gasification of coal to produce a pure hydrogen product. As the idea of hydrogen economy gains popularity, the focus on hydrogen as a replacement fuel source for hydrocarbons is increasing. Applications The WGSR is a highly valuable industrial reaction that is used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cativa Process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an iridium-containing catalyst, such as the complex r(CO)2I2sup>− (1). The Cativa and Monsanto processes are sufficiently similar that they can use the same chemical plant. Initial studies by Monsanto had shown iridium to be less active than rhodium for the carbonylation of methanol. Subsequent research, however, showed that the iridium catalyst could be promoted by ruthenium, and this combination leads to a catalyst that is superior to the rhodium-based systems. The switch from rhodium to iridium also allows the use of less water in the reaction mixture. This change reduces the number of drying columns necessary, decreases formation of by-products, such as propionic acid, and suppresses the water gas shift reaction. The catalytic cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Le Chatelier's Principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French chemist Henry Louis Le Chatelier, and sometimes also credited to Karl Ferdinand Braun, who discovered it independently. It can be stated as: Phenomena in apparent contradiction to Le Chatelier's principle can also arise in systems of simultaneous equilibrium (see response reactions). Le Chatelier's principle is sometimes alluded to in discussions of topics other than thermodynamics. Thermodynamic statement The Le Chatelier–Braun principle analyzes the qualitative behaviour of a thermodynamic system when a designated one of its externally controlled state variables, say L, changes by an amount \Delta L, the 'driving change', causing a change \delta_ M, the 'response of prime interest', in its conjugate state variable M, all other ext ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic Reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change Δ''G''⚬ is negative." A strongly exothermic reaction will usually also be exergonic because Δ''H''⚬ makes a major contribution to Δ''G''⚬. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Examples Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations. As an example in everyday life, hand warmers make use of the oxidation of iron to achieve an exothermic reaction: :4Fe + 3O2 → 2Fe2O3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
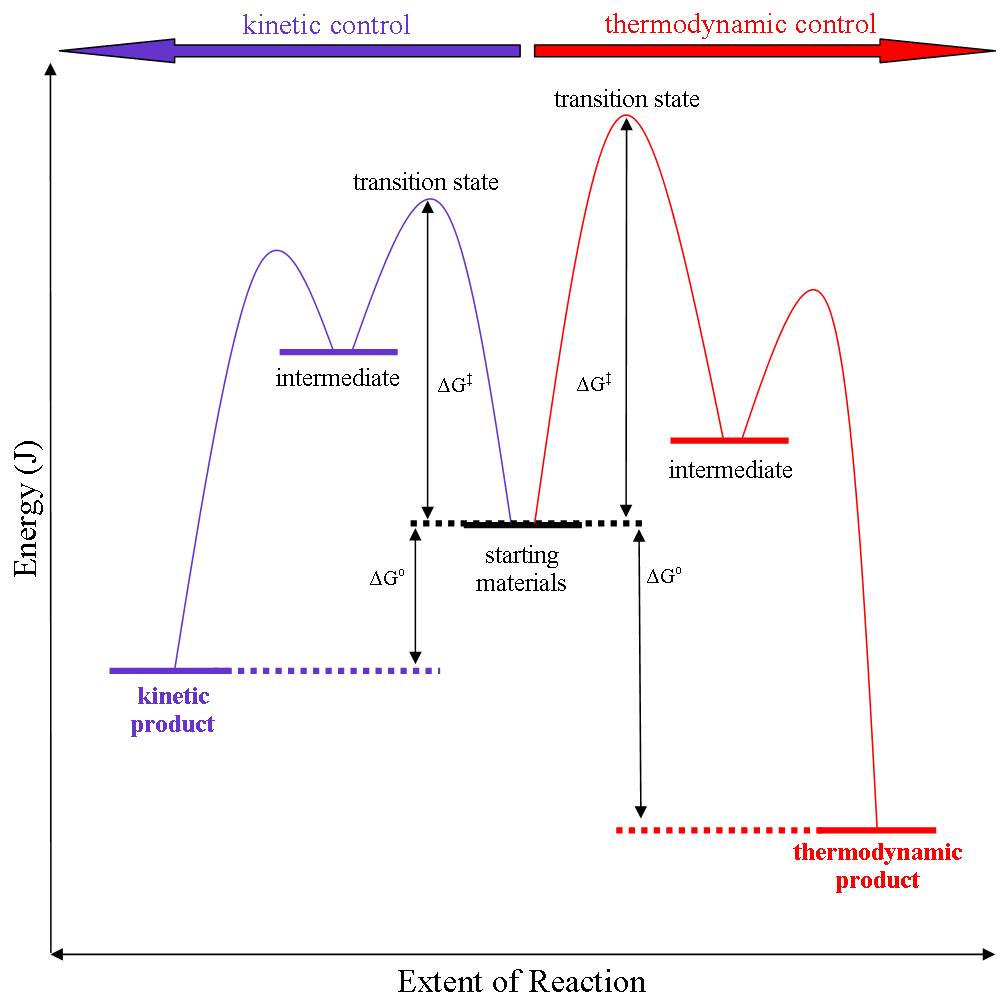
Thermodynamic Versus Kinetic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
K DGr WGS
K, or k, is the eleventh letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''kay'' (pronounced ), plural ''kays''. The letter K usually represents the voiceless velar plosive. History The letter K comes from the Greek letter Κ (kappa), which was taken from the Semitic kaph, the symbol for an open hand. This, in turn, was likely adapted by Semitic tribes who had lived in Egypt from the hieroglyph for "hand" representing /ḏ/ in the Egyptian word for hand, ⟨ ḏ-r-t⟩ (likely pronounced in Old Egyptian). The Semites evidently assigned it the sound value instead, because their word for hand started with that sound. K was brought into the Latin alphabet with the name ''ka'' /kaː/ to differentiate it from C, named ''ce'' (pronounced /keː/) and Q, named ''qu'' and pronounced /kuː/. In the earliest Latin inscriptions, the letters C, K and Q were all used to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principly used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII (in Germany alone half a million cars were built or rebuilt to run on wood gas). Production Syngas is produced by steam reforming or partial oxidation of natural gas or liquid hydrocarbons, or coal gasification. Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of methane: : In principle, but rarely in practice, biomass and related hydrocarbon feedstocks could be used to generate biogas and biochar in waste-to-energy gasification facilities. The gas generated (mostly methane and carbon dioxide) is sometimes described as ''syngas'' but its co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sorption Enhanced Water Gas Shift
Sorption enhanced water gas shift (SEWGS) is a technology that combines a pre-combustion carbon capture process with the water gas shift reaction (WGS) in order to produce a hydrogen rich stream from the syngas fed to the SEWGS reactor. The water gas shift reaction converts the carbon monoxide into carbon dioxide, according to the following chemical reaction: : CO + H2O CO2 + H2 While carbon dioxide is captured and removed through an adsorption process. The in-situ CO2 adsorption and removal shifts the water gas shift reaction to the right-hand side, thereby completely converting the CO and maximizing the production of high pressure hydrogen. Since the beginning of the second decade of the 21st century this technology has started gaining attention, as it shows advantages over carbon capture conventional technologies and because hydrogen is considered the energy carrier of the future. Process The SEWGS technology is the combination of the water gas shift reaction with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a '' surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term '' sorption'' encompasses both processes, while '' desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |