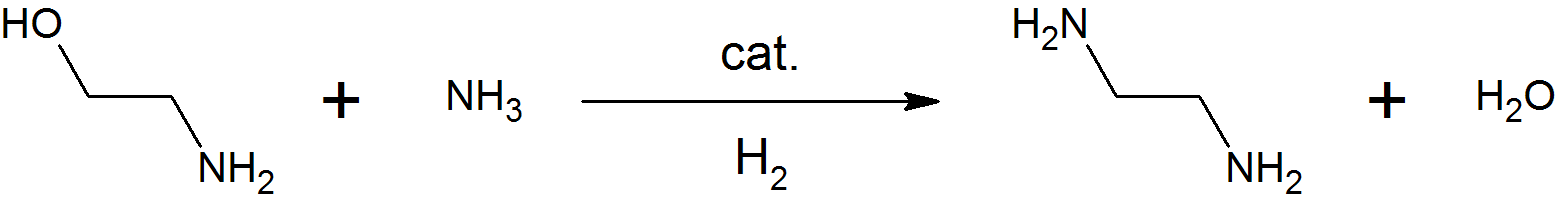|
Sorption Enhanced Water Gas Shift
Sorption enhanced water gas shift (SEWGS) is a technology that combines a pre-combustion Carbon capture and storage, carbon capture process with the Water-gas shift reaction, water gas shift reaction (WGS) in order to produce a hydrogen rich stream from the syngas fed to the SEWGS reactor. The Water-gas shift reaction, water gas shift reaction converts the carbon monoxide into carbon dioxide, according to the following chemical reaction: : CO + H2O CO2 + H2 While carbon dioxide is captured and removed through an adsorption process. The in-situ CO2 adsorption and removal shifts the water gas shift reaction to the right-hand side, thereby completely converting the CO and maximizing the production of high pressure hydrogen. Since the beginning of the second decade of the 21st century this technology has started gaining attention, as it shows advantages over carbon capture conventional technologies and because hydrogen is considered the energy carrier of the future. Process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Capture And Storage
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually the CO2 is captured from large point sources, such as a chemical plant or biomass power plant, and then stored in an underground geological formation. The aim is to prevent the release of CO2 from heavy industry with the intent of mitigating the effects of climate change. CO2 has been injected into geological formations for several decades for enhanced oil recovery and after separation from natural gas, but this has been criticised for producing more emissions when the gas or oil is burned. Carbon capture and utilization (CCU) and CCS are sometimes discussed collectively as carbon capture, utilization, and sequestration (CCUS). This is because CCS is a relatively expensive process yielding a product which is often too cheap. Hence, car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solutes
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Gas Combined Cycle
A combined cycle power plant is an assembly of heat engines that work in tandem from the same source of heat, converting it into mechanical energy. On land, when used to make electricity the most common type is called a combined cycle gas turbine (CCGT) plant. The same principle is also used for marine propulsion, where it is called a combined gas and steam (COGAS) plant. Combining two or more thermodynamic cycles improves overall efficiency, which reduces fuel costs. The principle is that after completing its cycle in the first engine, the working fluid (the exhaust) is still hot enough that a second subsequent heat engine can extract energy from the heat in the exhaust. Usually the heat passes through a heat exchanger so that the two engines can use different working fluids. By generating power from multiple streams of work, the overall efficiency can be increased by 50–60%. That is, from an overall efficiency of the system of say 34% for a simple cycle, to as much as 64% ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Economy
The hydrogen economy is using hydrogen to decarbonize economic sectors which are hard to electrify, essentially, the "hard-to-abate" sectors such as cement, steel, long-haul transport etc. In order to phase out fossil fuels and limit climate change, hydrogen can be created from water using renewable sources such as wind and solar, and its combustion only releases water vapor to the atmosphere. Hydrogen is an energetic fuel, frequently used as rocket fuel, but numerous technical challenges prevent the creation of a large-scale hydrogen economy. These include the difficulty of developing long-term storage, pipelines and engine equipment; a relative lack of off-the-shelf engine technology that can currently run safely on hydrogen; safety concerns regarding the high reactivity of hydrogen fuel with oxygen in ambient air; the expense of producing it by electrolysis; and a lack of efficient photochemical water splitting technology. Hydrogen can also react in a fuel cell, which effic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Global Warming
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to Earth's climate. The current rise in global average temperature is more rapid than previous changes, and is primarily caused by humans burning fossil fuels. Fossil fuel use, deforestation, and some agricultural and industrial practices increase greenhouse gases, notably carbon dioxide and methane. Greenhouse gases absorb some of the heat that the Earth radiates after it warms from sunlight. Larger amounts of these gases trap more heat in Earth's lower atmosphere, causing global warming. Due to climate change, deserts are expanding, while heat waves and wildfires are becoming more common. Increased warming in the Arctic has contributed to melting permafrost, glacial retreat and sea ice loss. Higher temperatures are also causing m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Diethanolamine
Methyl diethanolamine, also known as ''N''-methyl diethanolamine and more commonly as MDEA, is the organic compound with the formula CH3N(C2H4OH)2. It is a colorless liquid with an ammonia odor. It is miscible with water, ethanol and benzene. A tertiary amine, it is widely used as a sweetening agent in chemical, oil refinery, syngas production and natural gas.Matthias Frauenkron, Johann-Peter Melder, Günther Ruider, Roland Rossbacher, Hartmut Höke "Ethanolamines and Propanolamines" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. Similar compounds are monoethanolamine (MEA), a primary amine, and diethanolamine (DEA), a secondary amine, both of which are also used for amine gas treating. MDEA's defining characteristic when compared to these other amines is its ability to preferentially remove H2S (and strip CO2) from sour gas streams. MDEA's popularity as a solvent for gas treating stems from several advantages it has when compared to other alkanolamin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethanolamine
Triethanolamine, or TEA is a viscous organic compound that is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although samples may appear yellow because of impurities. Production Triethanolamine is produced from the reaction of ethylene oxide with aqueous ammonia, also produced are ethanolamine and diethanolamine. The ratio of the products can be controlled by changing the stoichiometry of the reactants. : Applications Triethanolamine is used primarily in making surfactants, such as for emulsifier. It is a common ingredient in formulations used for both industrial and consumer products. The triethanolamine neutralizes fatty acids, adjusts and pH buffer, buffers the pH, and solubilizes oils and other ingredients that are not completely Solubility, soluble in water. Triethanolammonium salts in some cases are more soluble than salts of alkali metals that might be us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethanolamine
Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encountered as a colorless, viscous liquid. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" ( Group 2B). Production The reaction of ethylene oxide with aqueous ammonia first produces ethanolamine: :C2H4O + NH3 → H2NCH2CH2OH which reacts with a second and third equivalent of ethylene oxide to give DEA and triethanolamine: :C2H4O + H2NCH2CH2OH → HN(CH2CH2OH)2 :C2H4O + H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanolamine
Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula or . The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia.. ETA molecules are a component in the formation of cellular membranes and are thus a molecular building block for life. It was thought to exist only on Earth and on certain asteroids, but in 2021 evidence was found that ETA molecules exist in interstellar space. Derivatives of ethanolamine are widespread in nature; e.g., lipids, as precursor of a variety of ''N''-acylethanolamines (NAEs), that modulate several animal and plant physiological processes such as seed germination, plant–pathogen interactions, chloroplast development and flowering, as well as precursor, combined with arachidonic acid 20: 4, ω-6), to form the endocannabinoid anandamide (AEA: ; 20:4, ω-6). The ethanolamines comprise a gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (chemistry)
In chemistry, absorption is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter some bulk phase – liquid or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface (as in the case for adsorption). A more common definition is that "Absorption is a chemical or physical phenomenon in which the molecules, atoms and ions of the substance getting absorbed enters into the bulk phase (gas, liquid or solid) of the material in which it is taken up." A more general term is ''sorption'', which covers absorption, adsorption, and ion exchange. Absorption is a condition in which something takes in another substance. In many processes important in technology, the chemical absorption is used in place of the physical process, e.g., absorption of carbon dioxide by sodium hydroxide – such acid-base processes do not follow the Nernst partition law (see: solubility). For ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |