|
Verrucarin A
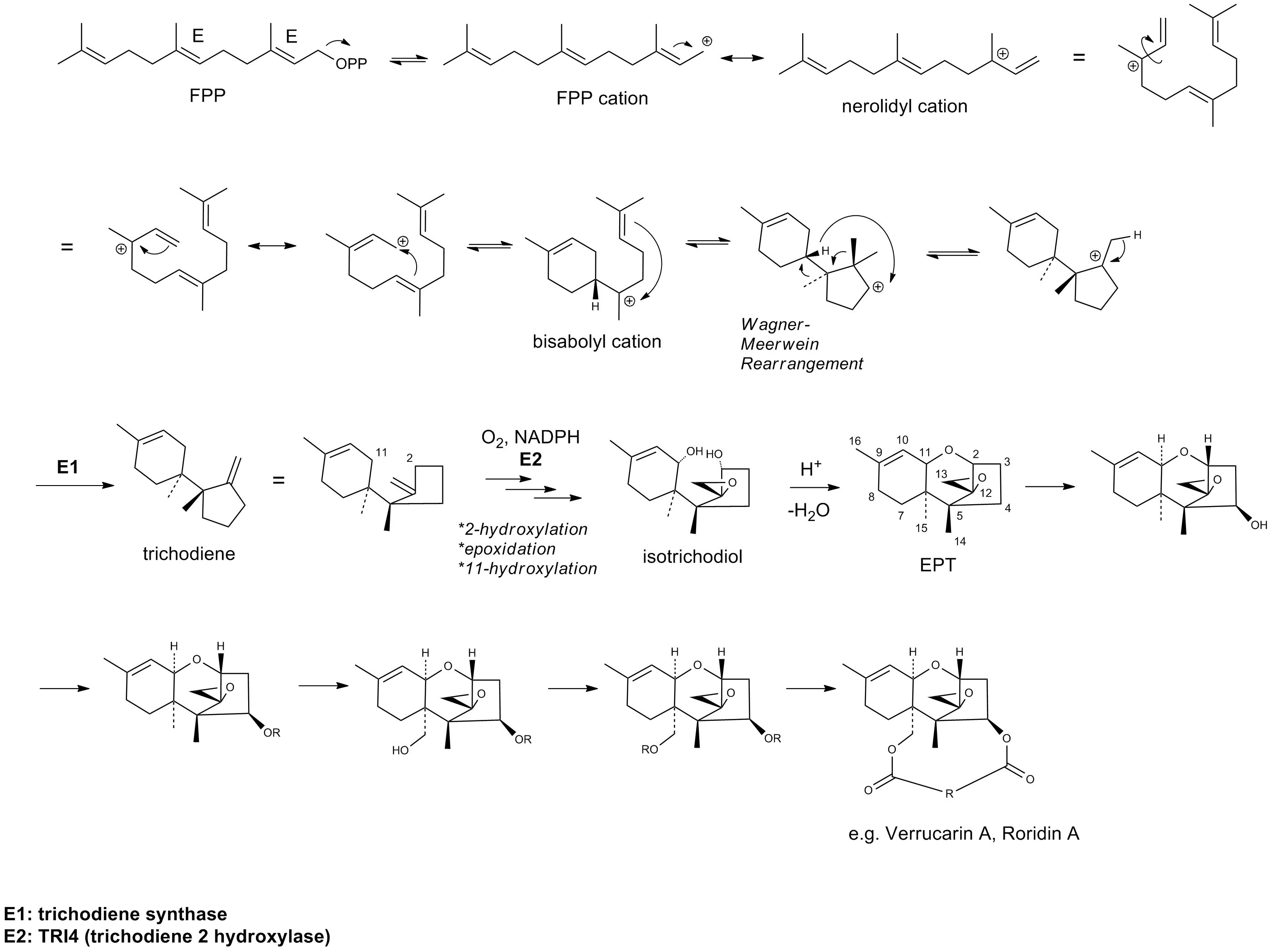
Verrucarin A is a chemical compound that belongs in the class of trichothecenes, a group of sesquiterpene toxins produced by several fungi, namely from the ''Fusarium'' species, that are responsible for infecting food grains. It was first described in 1962. Within the skeleton of the basic trichothecene structure, the olefin and epoxide are crucial for toxicity; ester functionalities and hydroxyl groups often contribute to the toxicity, thereby rendering verrucarin A as one of the most lethal examples. The mechanism of action for this class of toxins mainly inhibits protein biosynthesis by preventing peptidyl transferase activity. Although initially thought to be potentially useful as anticancer therapeutics, numerous examples of trichothecene derivatives were shown to be too toxic for clinical use. Biosynthesis Verrucarin A is classified as a type D trichothecene based on its substitution pattern in the 12,13-epoxytrichothec-9-ene (EPT) core structure. Type D differs from types A, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichothecenes
The trichothecenes are a large family of chemically related mycotoxins. They are produced by various species of ''Fusarium'', ''Myrothecium'', ''Trichoderma''/''Podostroma'', '' Trichothecium'', ''Cephalosporium'', '' Verticimonosporium'', and '' Stachybotrys''. Chemically, trichothecenes are a class of sesquiterpenes. The determining structural features causing the biological activity of trichothecenes are the 12,13-epoxy ring, the presence of hydroxyl or acetyl groups at appropriate positions on the trichothecene nucleus, and the structure and position of the side-chain. They are produced on many different grains such wheat, oats or maize by various ''Fusarium'' species including ''F. graminearum'', ''F. sporotrichioides'', ''F. poae'' and ''F. equiseti''. Some molds that produce trichothecene mycotoxins, for example '' Stachybotrys chartarum'', can grow in damp indoor environments. It has been found that macrocyclic trichothecenes produced by ''S. chartarum'' can become airborn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. Sesquiterpenes are found naturally in plants and insects, as semiochemicals, e.g. defensive agents or pheromones. Biosynthesis and examples The reaction of geranyl pyrophosphate with isopentenyl pyrophosphate results in the 15-carbon farnesyl pyrophosphate (FPP), which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene. Cyclic sesquiterpenes are more common than cyclic monoterpenes because of the increased chain length and additional double bond in the sesquiterpene precursors. In addition to common six-membered ring systems such as the ones found in zingiberene and bisacurone, cyclization of one end of the chain to the other end can l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fusarium
''Fusarium'' is a large genus of filamentous fungi, part of a group often referred to as hyphomycetes, widely distributed in soil and associated with plants. Most species are harmless saprobes, and are relatively abundant members of the soil microbial community. Some species produce mycotoxins in cereal crops that can affect human and animal health if they enter the food chain. The main toxins produced by these ''Fusarium'' species are fumonisins and trichothecenes. Despite most species apparently being harmless (some existing on the skin as commensal members of the skin flora), some ''Fusarium'' species and subspecific groups are among the most important fungal pathogens of plants and animals. The name of ''Fusarium'' comes from Latin ''fusus'', meaning a spindle. Taxonomy The taxonomy of the genus is complex. A number of different schemes have been used, and up to 1,000 species have been identified at times, with approaches varying between wide and narrow concepts of speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mevalonate Pathway
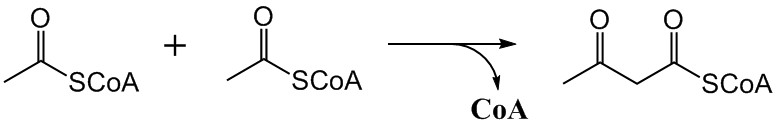
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance (pharmacology), clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Ronald W. Estabrook, Estabrook, David Y. Cooper, Cooper, and Otto Rosenthal, Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of secondary metabolite, defensive compounds, fatty acids, and hormones. CYP enzymes have been identified in all kingdom (biology), kingdoms of life: animals, plants, fungus, fungi, protists, bacteria, and archaea, as well as in viruses. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. , more than 300,000 distinct CYP proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis Of Verrucarin A
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g. NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |