|
Unbihexium
Unbihexium, also known as element 126 or eka-plutonium, is the hypothetical chemical element with atomic number 126 and placeholder symbol Ubh. ''Unbihexium'' and ''Ubh'' are the temporary IUPAC name and symbol, respectively, until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table, unbihexium is expected to be a g-block superactinide and the eighth element in the 8th period. Unbihexium has attracted attention among nuclear physicists, especially in early predictions targeting properties of superheavy elements, for 126 may be a magic number of protons near the center of an island of stability, leading to longer half-lives, especially for 310Ubh or 354Ubh which may also have magic numbers of neutrons. Early interest in possible increased stability led to the first attempted synthesis of unbihexium in 1971 and searches for it in nature in subsequent years. Despite several reported observations, more recent studies suggest that these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unbiquadium
Unbiquadium, also known as element 124 or eka-uranium, is the hypothetical chemical element with atomic number 124 and placeholder symbol Ubq. ''Unbiquadium'' and ''Ubq'' are the temporary IUPAC name and symbol, respectively, until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table, unbiquadium is expected to be a g-block superactinide and the sixth element in the 8th period. Unbiquadium has attracted attention, as it may lie within the island of stability, leading to longer half-lives, especially for 308Ubq which is predicted to have a magic number of neutrons (184). Despite several searches, unbiquadium has not been synthesized, nor have any naturally occurring isotopes been found to exist. It is believed that the synthesis of unbiquadium will be far more challenging than that of lighter undiscovered elements, and nuclear instability may pose further difficulties in identifying unbiquadium, unless the island of stability has a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flerovium
Flerovium is a superheavy chemical element with symbol Fl and atomic number 114. It is an extremely radioactive synthetic element. It is named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered in 1998. The lab's name, in turn, honours Russian physicist Georgy Flyorov ( in Cyrillic, hence the transliteration of " yo" to "e"). IUPAC adopted the name on 30 May 2012. The name and symbol had previously been proposed for element 102 (nobelium), but was not accepted by IUPAC at that time. It is a transactinide in the p-block of the periodic table. It is in period 7; the heaviest known member of the carbon group, and the last element whose chemistry has been investigated. Initial chemical studies in 2007–2008 indicated that flerovium was unexpectedly volatile for a group 14 element; in preliminary results it even seemed to exhibit properties similar to noble gases. More recent results show ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magic Number (physics)
In nuclear physics, a magic number is a number of nucleons (either protons or neutrons, separately) such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126 . For protons, this corresponds to the elements helium, oxygen, calcium, nickel, tin, lead and the hypothetical unbihexium, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy per nucleon than one would expect based upon predictions such as the semi-empirical mass formula and are hence more stable against nuclear decay. The unusual stability of isotopes having magic numbers means that transuranium elements could theoretically be created with extremely large nuclei and yet not be subject to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the "relative isotopic mass"), is within 1% of the whole number ''A''. Atoms with the same atomic number but dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. It is the 25th-most abundant element, making up 66 ppm of the Ear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of California At Davis
The University of California, Davis (UC Davis, UCD, or Davis) is a public land-grant research university near Davis, California. Named a Public Ivy, it is the northernmost of the ten campuses of the University of California system. The institution was first founded as an agricultural branch of the system in 1905 and became the seventh campus of the University of California in 1959. The university is classified among "R1: Doctoral Universities – Very high research activity". The UC Davis faculty includes 23 members of the National Academy of Sciences, 30 members of the American Academy of Arts and Sciences, 17 members of the American Law Institute, 14 members of the Institute of Medicine, and 14 members of the National Academy of Engineering. Among other honors that university faculty, alumni, and researchers have won are two Nobel Prizes, one Fields Medal, a Presidential Medal of Freedom, three Pulitzer Prizes, three MacArthur Fellowships, and a National Medal of Science. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-rays
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 Picometre, picometers to 10 Nanometre, nanometers, corresponding to frequency, frequencies in the range 30 Hertz, petahertz to 30 Hertz, exahertz ( to ) and energies in the range 145 electronvolt, eV to 124 keV. X-ray wavelengths are shorter than those of ultraviolet, UV rays and typically longer than those of gamma rays. In many languages, X-radiation is referred to as Röntgen radiation, after the German scientist Wilhelm Röntgen, Wilhelm Conrad Röntgen, who discovered it on November 8, 1895. He named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . Spellings of ''X-ray(s)'' in English include the variants ''x-ray(s)'', ''xray(s)'', and ''X ray(s)''. The most familiar use of X-ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-decay Stable Isobars
Beta-decay stable isobars are the set of nuclides which cannot undergo beta decay, that is, the transformation of a neutron to a proton or a proton to a neutron within the nucleus. A subset of these nuclides are also stable with regards to double beta decay or theoretically higher simultaneous beta decay, as they have the lowest energy of all nuclides with the same mass number. This set of nuclides is also known as the line of beta stability, a term already in common use in 1965. This line lies along the bottom of the nuclear valley of stability. Introduction The line of beta stability can be defined mathematically by finding the nuclide with the greatest binding energy for a given mass number, by a model such as the classical semi-empirical mass formula developed by C. F. Weizsäcker. These nuclides are local maxima in terms of binding energy for a given mass number. All odd mass numbers have only one beta decay stable nuclide. Among even mass number, six (124, 130, 136, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three carbonate-fluoride minerals, which includes bastnäsite-( Ce) with a formula of (Ce, La)CO3F, bastnäsite-( La) with a formula of (La, Ce)CO3F, and bastnäsite-( Y) with a formula of (Y, Ce)CO3F. Some of the bastnäsites contain OH− instead of F− and receive the name of hydroxylbastnasite. Most bastnäsite is bastnäsite-(Ce), and cerium is by far the most common of the rare earths in this class of minerals. Bastnäsite and the phosphate mineral monazite are the two largest sources of cerium and other rare-earth elements. Bastnäsite was first described by the Swedish chemist Wilhelm Hisinger in 1838. It is named for the Bastnäs mine near Riddarhyttan, Västmanland, Sweden. Bastnäsite also occurs as very high-quality specimens at the Zagi Mountains, Pakistan. Bastnäsite occurs in alkali granite and syenite and in associated pegmatites. It also occurs in carbonatites and in associated fenites and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
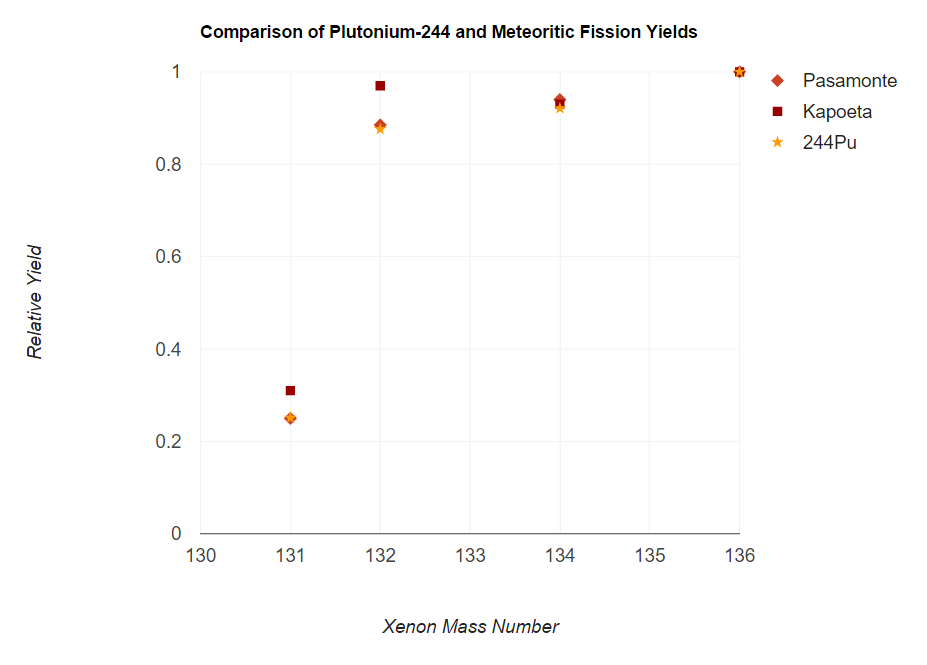
Plutonium-244
Plutonium-244 (244Pu) is an isotope of plutonium that has a half-life of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any other actinide isotope except for the three naturally abundant ones: uranium-235 (704 million years), uranium-238 (4.468 billion years), and thorium-232 (14.05 billion years). Although studies are in conflict, given the mathematics of the decay of plutonium-244, an exceedingly small amount should still be present in the Earth's composition, making plutonium a likely although unproven candidate as the shortest lived primordial element. Natural occurrence Accurate measurements, beginning in the early 1970s, have detected primordial plutonium-244, making it the shortest-lived primordial nuclide. The amount of 244Pu in the pre-Solar nebula (4.57×109 years ago) was estimated as 0.8% the amount of 238U. As the age of the Earth is about 57 half-lives of 244Pu, the amount of plutonium-244 left should be very small; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare Earth Element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)