|
UV-absorber
In polymer chemistry photo-oxidation (sometimes: oxidative photodegradation) is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break (chain scission), resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering. Technologies have been developed to both accelerate and inhibit this process. For example, plastic building components like doors, window frames and gutters are expected to last for decades, requiring the use of advanced UV-polymer stabilizers. Conversely, single-use plastics can be treated with biodegradable additives to accelerate their fragmentation. Many pigments and dyes can similarly have effects due to their ability to absorb UV-energy. Susceptible polymers Susceptibility to ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photodegradation
Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroys paintings and other artifacts. It is, however, partly responsible for remineralization of biomass and is used intentionally in some disinfection technologies. Photodegradation does not apply to how materials may be aged or degraded via infrared light or heat, but does include degradation in all of the ultraviolet light wavebands. Applications Foodstuffs The protection of food from photodegradation is very important. Some nutrients, for example, are affected by degradation when exposed to sunlight. In the case of beer, UV radiation causes a process that entails the degradation of hop bitter compounds to 3-methyl-2-buten-1-thiol and therefore changes the taste. As amber-colored glass has the ability to absorb UV radiation, beer bottles ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours. Technologies have been developed to both inhibit or promote degradation. For instance, polymer stabilizers ensure plastic items are produced with the desired properties, extend their useful lifespans, and facilitate their recycling. Conversely, biodegradable additives accelerate the degradation of plastic waste by improving its biodegradability. Some forms of plastic recycling can involve the complete degradation of a polymer back into monomers or other chemicals. In general, the effec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylate Polymer
An acrylate polymer (also known as acrylic or polyacrylate) is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity. Acrylate polymer is commonly used in cosmetics, such as nail polish, as an adhesive. History The first synthesis of acrylic polymer was reported by G.W.A Kahlbaum in 1880. Acrylic elastomers Acrylic elastomer is a general term for a type of synthetic rubber whose primary component is acrylic acid alkylester ( ethyl or butyl ester). Acrylic elastomer possesses characteristics of heat and oil resistance, with the ability to withstand temperatures of 170–180 °C. It is used primarily for producing oil seals and packaging related to automobiles. Acrylic elastomer can generally be characterized as one of two types. "Old" types include ACM (copolymer of acrylic acid ester and 2-chloroethyl vinyl ether) containing chlorine and ANM (copolymer of acrylic acid este ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autocatalytic
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 1999) p.151-2 Such a reaction is called an autocatalytic reaction. A ''set'' of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see autocatalytic set). Chemical reactions A chemical reaction of two reactants and two products can be written as : \alpha A + \beta B \rightleftharpoons \sigma S + \tau T where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organic compounds in air at ambient temperatures. Many common phenomena can be attributed to autoxidation, such as food going rancid, the 'drying' of varnishes and paints, and the perishing of rubber. It is also an important concept in both industrial chemistry and biology. Autoxidation is therefore a fairly broad term and can encompass examples of photooxygenation and catalytic oxidation. The common mechanism is a free radical chain reaction, where the addition of oxygen gives rise to hydroperoxides and their associated peroxy radicals (ROO•). Typically, an induction period is seen at the start where there is little activity; this is followed by a gradually accelerating take-up of oxygen, giving an autocatalytic reaction which can only be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain Reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events. Chain reactions are one way that systems which are not in thermodynamic equilibrium can release energy or increase entropy in order to reach a state of higher entropy. For example, a system may not be able to reach a lower energy state by releasing energy into the environment, because it is hindered or prevented in some way from taking the path that will result in the energy release. If a reaction results in a small energy release making way for more energy releases in an expanding chain, then the system will typically collapse explosively until much or all of the stored energy has been released. A macroscopic metaphor for chain reactions is thus a snowball causing a larger snowball until finally an avalanche results ("snowball effect"). This is a result of stored gravitation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing. Ageing Ailments of unknown cause Biogerontology Biological processes Causes of death Cellular processes Gerontology Life extension Metabolic disorders Metabolism Old age Time in life Wikipedia categories named after diseases and disorders {{CatAutoTOC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bottles, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'': low-density polyethylene is extruded using high pressure () and high temperature (), while high-density polyethylene is extruded using low pressure () and low temperature (). Polyethylene is usually thermoplastic, but it can be modified to become thermosetting instead, for example, in cross-linked polyethylene. History Polyethylene was first synthesized by the German chemist Hans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
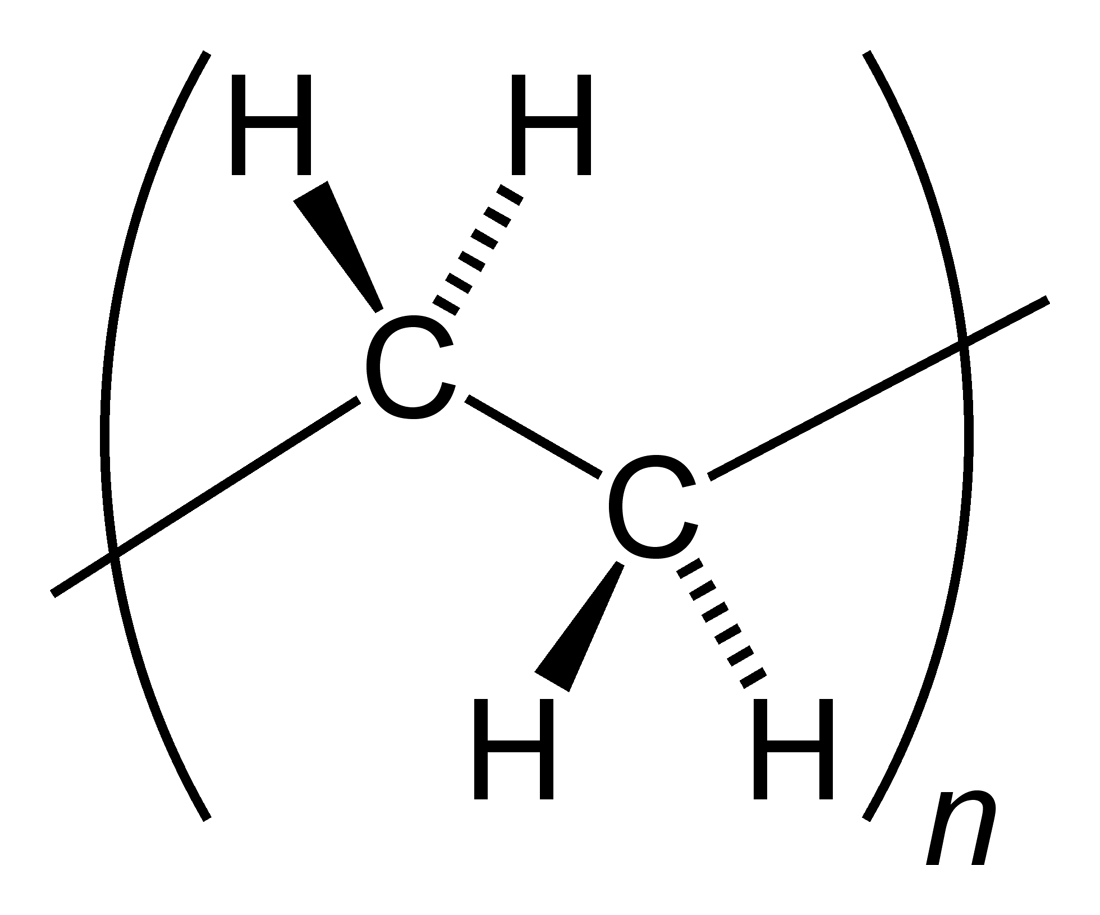
Polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene. Polypropylene belongs to the group of polyolefins and is partially crystalline and non-polar. Its properties are similar to polyethylene, but it is slightly harder and more heat-resistant. It is a white, mechanically rugged material and has a high chemical resistance. Bio-PP is the bio-based counterpart of polypropylene (PP). Polypropylene is the second-most widely produced commodity plastic (after polyethylene). In 2019, the global market for polypropylene was worth $126.03 billion. Revenues are expected to exceed US$145 billion by 2019. The sales of this material are forecast to grow at a rate of 5.8% per year until 2021. History Phillips Petroleum chemists J. Paul Hogan and Robert Banks first demonstrated the polymerization of propylene in 1951. The stereoselective polymerization t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyolefins
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds ( polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |