|
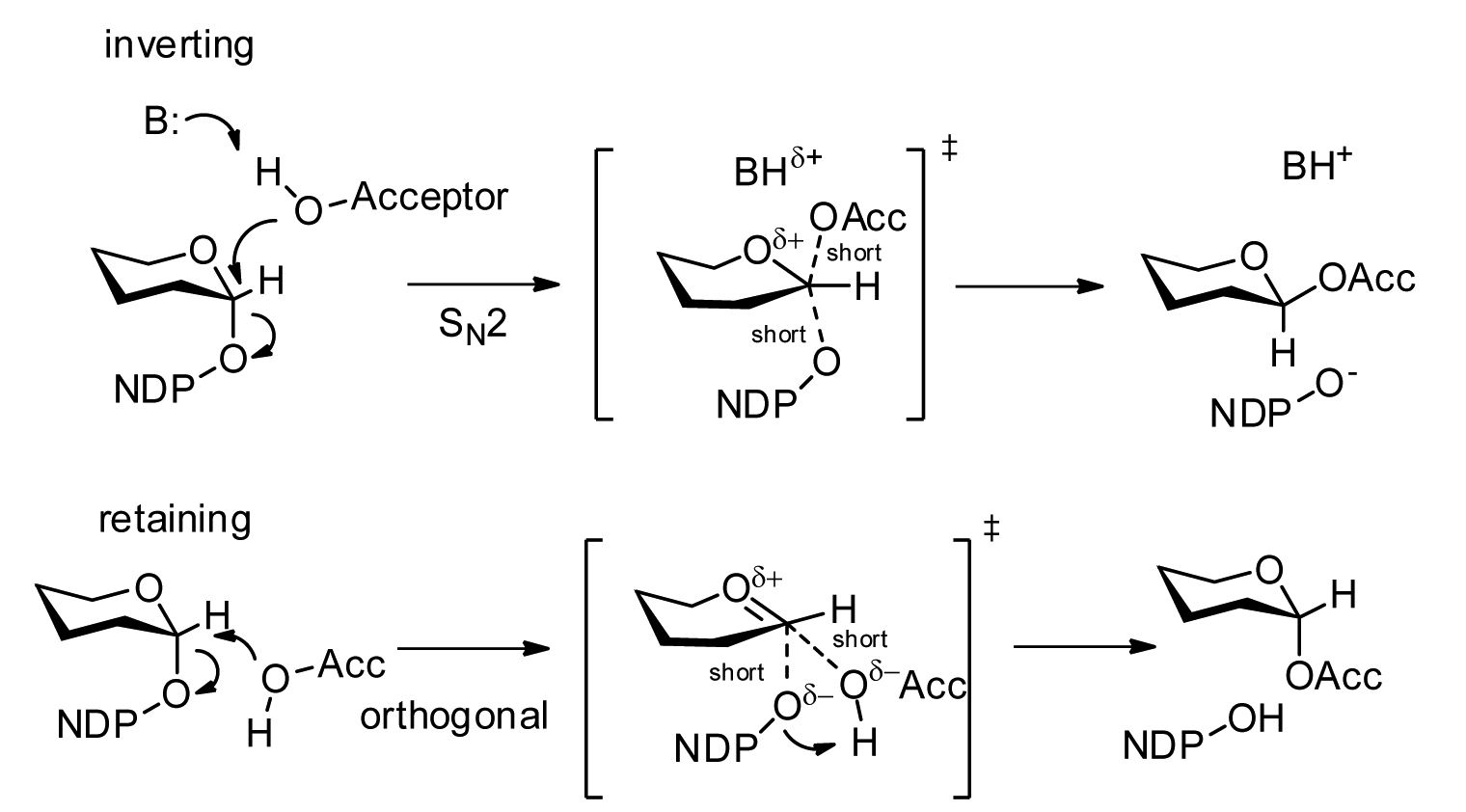
UDP-galactose 4′-epimerase
The enzyme UDP-glucose 4-epimerase (), also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity. Additionally, human and some bacterial GALE isoforms reversibly catalyze the formation of UDP-''N''-acetylgalactosamine (UDP-GalNAc) from UDP-''N''-acetylglucosamine ( UDP-GlcNAc) in the presence of NAD+, an initial step in glycoprotein or glycolipid synthesis. Historical significance Dr. Luis Leloir deduced the role of GALE in galactose metabolism during his tenure at the Instituto de Investigaciones Bioquímicas del Fundación Campomar, initially terming the enzyme waldenase. Dr. Leloir was awarded the 1970 Nobel Prize in Chemistry for his discovery ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and NADH, reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, can occur in the Great Oxygenation Event, oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal ions, meaning this is a plausible prebiotic pathway for abiogenesis. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Kar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EC 5
EC or ec may refer to: Arts and entertainment * EC Comics, an American publisher of comic books * ''Electric Circus'', a Canadian television program * Eric Clapton Stratocaster, signature model guitars by Fender Businesses and organisations Government * Environment and Climate Change Canada, a Canadian federal government department * European Commission, the executive body of the European Union * European Council, the European Union institution comprising the college of heads of state of government * European Communities, one of the three pillars of the EU * European Community, a significant component of the European Union from 1993 to 2009, renamed European Economic Community Transportation * EuroCity, a train service of the European inter-city rail network * EasyJet Europe (IATA code: EC) * Avialeasing (former IATA code: EC), a cargo airline * East Coast (train operating company), a train operating company in the UK * EC, the aircraft registration prefix for Spain Educatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactosemia
Galactosemia (British galactosaemia, from Greek γαλακτόζη + αίμα, meaning galactose + blood, accumulation of galactose in blood) is a rare genetics, genetic Metabolism, metabolic Disease, disorder that affects an individual's ability to metabolize the sugar galactose properly. Galactosemia follows an autosomal recessive mode of inheritance that confers a deficiency in an enzyme responsible for adequate galactose degradation. Friedrich Goppert (1870–1927), a German physician, first described the disease in 1917, with its cause as a defect in galactose metabolism being identified by a group led by Herman Kalckar in 1956. Galactosemia was the second disorder found to be detectable through newborn screening methods by Robert Guthrie. Its incidence is about 1 per 60,000 births for people of European ancestry. In other populations the incidence rate differs. Galactosemia is about one hundred times more common (1:480 births) in the Irish Traveller population. Symptoms and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosyltransferases
Glycosyltransferases (GTFs, Gtfs) are enzymes ( EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based. The result of glycosyl transfer can be a carbohydrate, glycoside, oligosaccharide, or a polysaccharide. Some glycosyltransferases catalyse transfer to inorganic phosphate or water. Glycosyl transfer can also occur to protein residues, usually to tyrosine, serine, or threonine to give O-linked glycoproteins, or to asparagine to give N-linked glycoproteins. Mannosyl groups may be transferred to tryptophan to generate C-mannosyl tryptophan, which is relatively abundant in eukaryotes. Transferases may also use lipids as an acceptor, forming glycolipids, and even use lipid-linked sugar phosphate donors, such as dolichol phosphates in eukaryoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inositol-3-phosphate Synthase
In enzymology, an inositol-3-phosphate synthase () is an enzyme that catalyzes the chemical reaction :D-glucose 6-phosphate \rightleftharpoons 1D-myo-inositol 3-phosphate Hence, this enzyme has one substrate, D-glucose 6-phosphate, and one product, 1D-myo-inositol 3-phosphate. This enzyme belongs to the family of isomerases, specifically the class of intramolecular lyases. The systematic name of this enzyme class is 1D-myo-inositol-3-phosphate lyase (isomerizing). Other names in common use include myo-inositol-1-phosphate synthase, D-glucose 6-phosphate cycloaldolase, inositol 1-phosphate synthatase, glucose 6-phosphate cyclase, inositol 1-phosphate synthetase, glucose-6-phosphate inositol monophosphate cycloaldolase, glucocycloaldolase, and 1L-myo-inositol-1-phosphate lyase (isomerizing). This enzyme participates in streptomycin biosynthesis and inositol phosphate metabolism. It employs one cofactor, NAD+. The reaction this enzyme catalyses represents the first committed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inositol-1-phosphate
Inositol phosphates are a group of mono- to hexaphosphorylated inositols. Each form of inositol phosphate is distinguished by the number and position of the phosphate group on the inositol ring. * inositol monophosphate (IP) * inositol bisphosphate (IP2) * inositol trisphosphate (IP3) * inositol tetrakisphosphate (IP4) * inositol pentakisphosphate (IP5) * inositol hexaphosphate (IP6) also known as phytic acid, or phytate (as a salt). A series of phosphorylation and dephosphorylation reactions are carried out by at least 19 phosphoinositide kinases and 28 phosphoinositide phosphatase enzymes allowing for the inter-conversion between the inositol phosphate compounds based on cellular demand. Inositol phosphates play a crucial role in various signal transduction pathways responsible for cell growth and differentiation, apoptosis, DNA repair, RNA export, regeneration of ATP and more. Functions Inositol trisphosphate The inositol-phospholipid signaling pathway is responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoglucomutase
Phosphoglucomutase () is an enzyme that transfers a phosphate group on an α-D-glucose monomer from the 1 to the 6 position in the forward direction or the 6 to the 1 position in the reverse direction. More precisely, it facilitates the interconversion of glucose 1-phosphate and glucose 6-phosphate. Function Role in glycogenolysis After glycogen phosphorylase catalyzes the phosphorolytic cleavage of a glucosyl residue from the glycogen polymer, the freed glucose has a phosphate group on its 1-carbon. This glucose 1-phosphate molecule is not itself a useful metabolic intermediate, but phosphoglucomutase catalyzes the conversion of this glucose 1-phosphate to glucose 6-phosphate (see below for the mechanism of this reaction). Glucose 6-phosphate’s metabolic fate depends on the needs of the cell at the time it is generated. If the cell is low on energy, then glucose 6-phosphate will travel down the glycolytic pathway, eventually yielding two molecules of adenosine tripho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose-6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way. Because of its prominent position in cellular chemistry, glucose 6-phosphate has many possible fates within the cell. It lies at the start of two major metabolic pathways: glycolysis and the pentose phosphate pathway. In addition to these two metabolic pathways, glucose 6-phosphate may also be converted to glycogen or starch for storage. This storage is in the liver and muscles in the form of glycogen for most multicellular animals, and in intracellular starch or glycogen granules for most other organisms. Production From glucose Within a cell, glucose 6-phosphate is produced by phosphorylation of glucose on the sixth carbon. This is catalyzed by the enzyme hexokinase in most cells, and, in higher animals, glucokinase in certain cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose-1-phosphate Uridyltransferase
Galactose-1-phosphate uridyltransferase (or GALT, G1PUT) is an enzyme () responsible for converting ingested galactose to glucose. Galactose-1-phosphate uridyltransferase (GALT) catalyzes the second step of the Leloir pathway of galactose metabolism, namely: :UDP-glucose + galactose 1-phosphate \rightleftharpoons glucose 1-phosphate + UDP-galactose The expression of GALT is controlled by the actions of the FOXO3 gene. The absence of this enzyme results in classic galactosemia in humans and can be fatal in the newborn period if lactose is not removed from the diet. The pathophysiology of galactosemia has not been clearly defined. Image:DGalactose Fischer.svg, galactose Image:D-glucose chain (Fischer).svg, glucose Mechanism GALT catalyzes the second reaction of the Leloir pathway of galactose metabolism through ping pong bi-bi kinetics with a double displacement mechanism. This means that the net reaction consists of two reactants and two products (see the reaction above) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose-1-phosphate
D-Galactose-1-phosphate is an intermediate in the intraconversion of glucose and uridine diphosphate galactose. It is formed from galactose by galactokinase.The improper metabolism of galactose-1-phosphate is a characteristic of galactosemia. The Leloir pathway is responsible for such metabolism of galactose and its intermediate, galactose-1-phosphate. Deficiency of enzymes found in this pathway can result in galactosemia; therefore, diagnosis of this genetic disorder occasionally involves measuring the concentration of these enzymes. One of such enzymes is galactose-1-phosphate uridylyltransferase (GALT). The enzyme catalyzes the transfer of a UDP-activator group from UDP-glucose to galactose-1-phosphate. Although the cause of enzyme deficiency in the Leloir pathway is still disputed amongst researchers, some studies suggest that protein misfolding of GALT, which may lead to an unfavorable conformational change that impacts its thermal stability and substrate-binding affinity, may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose Mutarotase
Galactose mutarotase (aldose 1-epimerase) (gene name GALM) is a human enzyme that reversibly converts α-aldose to the β-anomer. This enzyme catalyzes the first step of the Leloir pathway, which is involved in galactose metabolism. It belongs to family of aldose An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ket ... epimerases. The two main amino acids in the enzyme active site are Glu 304, which acts as a Bronsted-Lowry base and abstracts a proton, and His 170, which acts as Bronsted-Lowry Acid to donate a proton to the galactose. References External links PDBe-KB provides an overview of all the structure information available in the PDB for Human Aldose 1-epimerase (Galactose mutarotase) {{gene-2-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |