|
Typhim Vi
The Vi capsular polysaccharide vaccine (or ViCPS) is a typhoid vaccine recommended by the World Health Organization for the prevention of typhoid (another is Ty21a). The vaccine was first licensed in the US in 1994 and is made from the purified Vi capsular polysaccharide from the Ty2 ''Salmonella'' Typhi strain; it is a subunit vaccine. Medical uses The vaccine may be used in endemic areas in order to prevent typhoid. It is also commonly used to protect people who are traveling to parts of the world where typhoid is endemic. Dosing The vaccine is injected either under the skin or into a muscle at least seven days before traveling to the typhoid-affected area (the CDC recommend 14 days). The vaccine is not effective in children under the age of two. To maintain immunity, the vaccine should be repeated every three years. Efficacy and duration of protection The vaccine offers effective protection the first year after being given (with between 50% and 80% efficacy) and the se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Typhoid
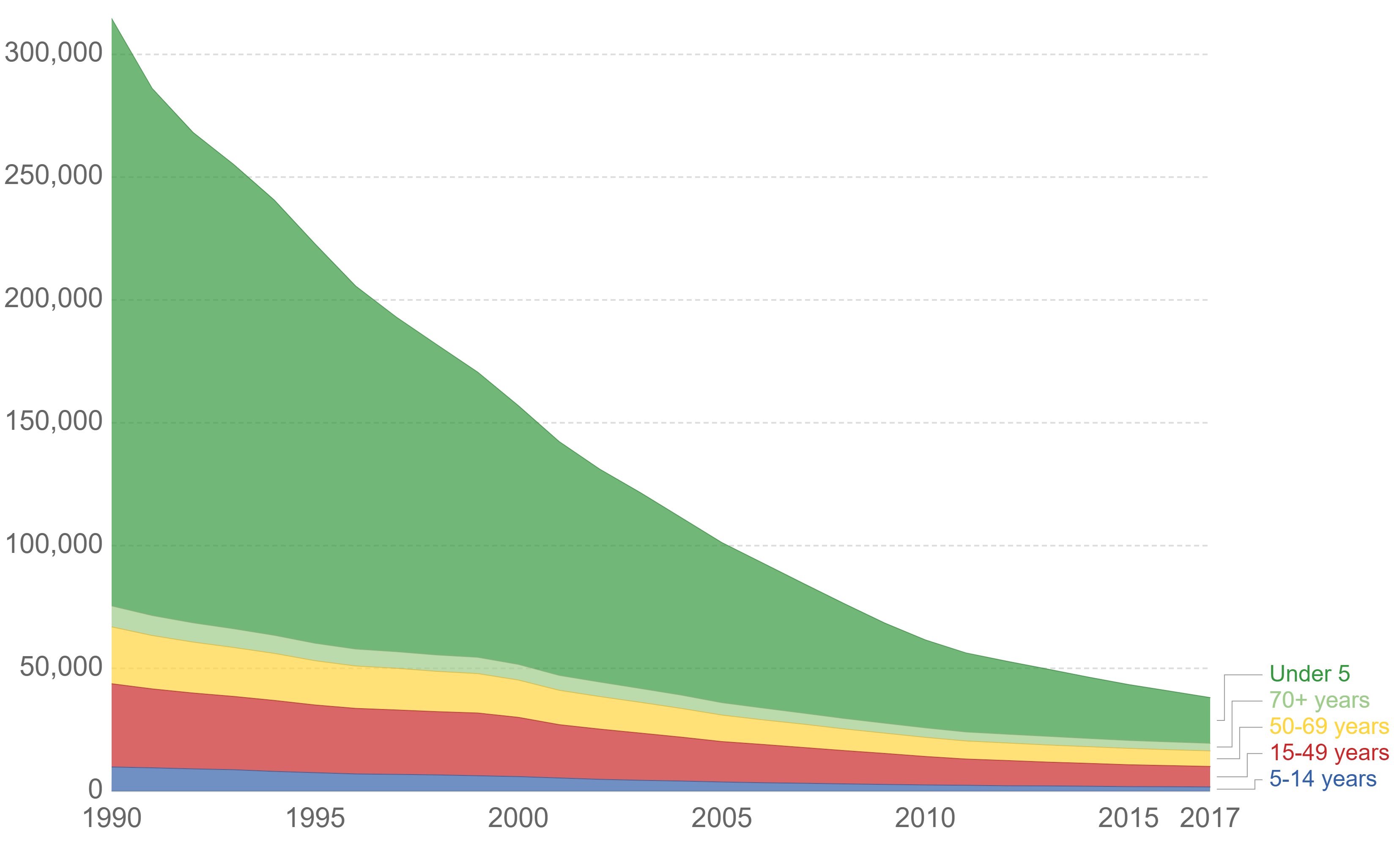
Typhoid fever, also known as typhoid, is a disease caused by '' Salmonella'' serotype Typhi bacteria. Symptoms vary from mild to severe, and usually begin six to 30 days after exposure. Often there is a gradual onset of a high fever over several days. This is commonly accompanied by weakness, abdominal pain, constipation, headaches, and mild vomiting. Some people develop a skin rash with rose colored spots. In severe cases, people may experience confusion. Without treatment, symptoms may last weeks or months. Diarrhea may be severe, but is uncommon. Other people may carry the bacterium without being affected, but they are still able to spread the disease. Typhoid fever is a type of enteric fever, along with paratyphoid fever. ''S. enterica'' Typhi is believed to infect and replicate only within humans. Typhoid is caused by the bacterium ''Salmonella enterica'' subsp. ''enterica'' serovar Typhi growing in the intestines, peyers patches, mesenteric lymph nodes, spleen, liver, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Typherix
The Vi capsular polysaccharide vaccine (or ViCPS) is a typhoid vaccine recommended by the World Health Organization for the prevention of typhoid (another is Ty21a). The vaccine was first licensed in the US in 1994 and is made from the purified Vi capsular polysaccharide from the Ty2 ''Salmonella'' Typhi strain; it is a subunit vaccine. Medical uses The vaccine may be used in endemic areas in order to prevent typhoid. It is also commonly used to protect people who are traveling to parts of the world where typhoid is endemic. Dosing The vaccine is injected either under the skin or into a muscle at least seven days before traveling to the typhoid-affected area (the CDC recommend 14 days). The vaccine is not effective in children under the age of two. To maintain immunity, the vaccine should be repeated every three years. Efficacy and duration of protection The vaccine offers effective protection the first year after being given (with between 50% and 80% efficacy) and the se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Typhoid Vaccine
Typhoid vaccines are vaccines that prevent typhoid fever. Several types are widely available: typhoid conjugate vaccine (TCV), Ty21a (a live oral vaccine) and Vi capsular polysaccharide vaccine (ViPS) (an injectable subunit vaccine). They are about 30 to 70% effective in the first two years, depending on the specific vaccine in question. The Vi-rEPA vaccine has been shown to be efficacious in children. The World Health Organization (WHO) recommends vaccinating all children in areas where the disease is common. Otherwise they recommend vaccinating those at high risk. Vaccination campaigns can also be used to control outbreaks of disease. Depending on the vaccine, additional doses are recommended every three to seven years. In the United States the vaccine is only recommended in those at high risk such as travelers to areas of the world where the disease is common. The vaccines available as of 2018 are very safe. Minor side effects may occur at the site of injection. The injecta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetanus Toxoid
Tetanus vaccine, also known as tetanus toxoid (TT), is a toxoid vaccine used to prevent tetanus. During childhood, five doses are recommended, with a sixth given during adolescence. After three doses, almost everyone is initially immune, but additional doses every ten years are recommended to maintain immunity. A booster shot should be given within 48 hours of an injury to people whose immunization is out of date. For people with high-risk injuries who are not fully immunized, tetanus antitoxin may also be recommended. Confirming that pregnant women are up to date on tetanus immunization during each pregnancy can prevent both maternal and neonatal tetanus. The vaccine is very safe, including during pregnancy and in those with HIV/AIDS. Redness and pain at the site of injection occur in between 25% and 85% of people. Fever, feeling tired, and minor muscle pain occurs in less than 10% of people. Severe allergic reactions occur in less than one in 100,000 people. A number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Typhoid Conjugate Vaccine
The Vi capsular polysaccharide vaccine (or ViCPS) is a typhoid vaccine recommended by the World Health Organization for the prevention of typhoid (another is Ty21a). The vaccine was first licensed in the US in 1994 and is made from the purified Vi capsular polysaccharide from the Ty2 ''Salmonella'' Typhi strain; it is a subunit vaccine. Medical uses The vaccine may be used in endemic areas in order to prevent typhoid. It is also commonly used to protect people who are traveling to parts of the world where typhoid is endemic. Dosing The vaccine is injected either under the skin or into a muscle at least seven days before traveling to the typhoid-affected area (the CDC recommend 14 days). The vaccine is not effective in children under the age of two. To maintain immunity, the vaccine should be repeated every three years. Efficacy and duration of protection The vaccine offers effective protection the first year after being given (with between 50% and 80% efficacy) and the se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exotoxin A
The Pseudomonas exotoxin (or exotoxin A) is an exotoxin produced by ''Pseudomonas aeruginosa''. ''Vibrio cholerae'' produces a similar protein called the Cholix toxin (). It inhibits elongation factor-2. It does so by ADP-ribosylation of EF2 using NAD+. This then causes the elongation of polypeptides to cease. This mechanism is similar to that of diphtheria toxin. It has been investigated as a treatment for hepatitis B Hepatitis B is an infectious disease caused by the '' Hepatitis B virus'' (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection. Many people have no symptoms during an initial infection. F ... and cancer. References External links *P11439 (eta)in InterPro domain view {{Toxins Bacterial toxins Proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudomonas Aeruginosa
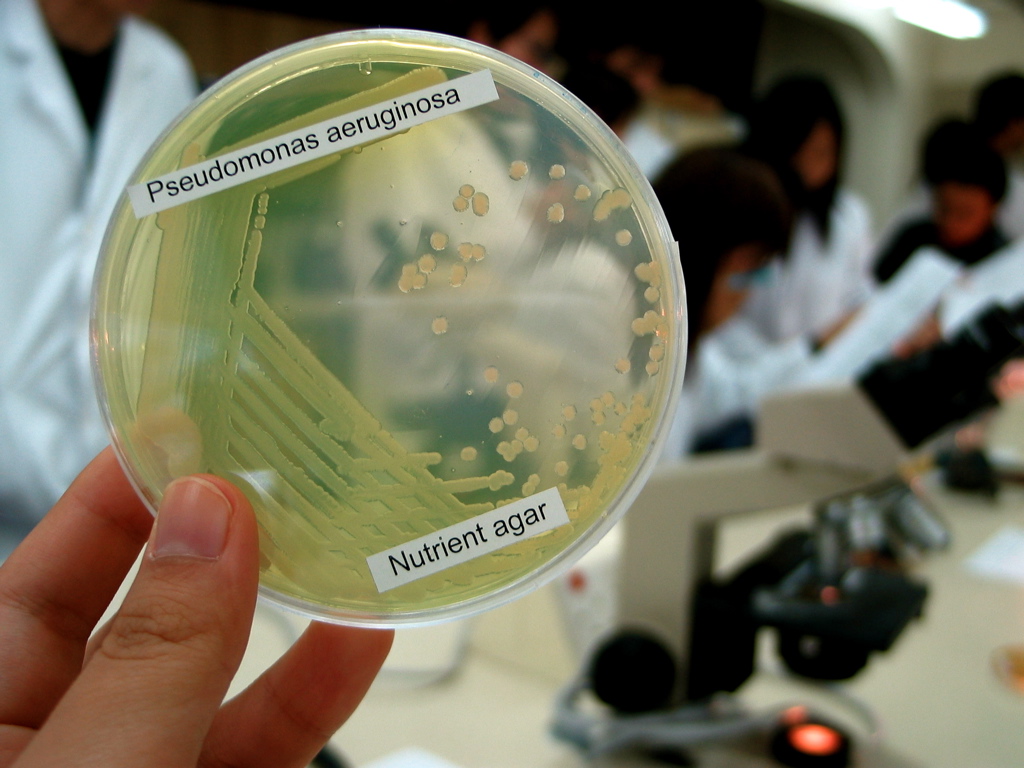
''Pseudomonas aeruginosa'' is a common encapsulated, gram-negative, aerobic– facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, ''P. aeruginosa'' is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. The organism is considered opportunistic insofar as serious infection often occurs during existing diseases or conditions – most notably cystic fibrosis and traumatic burns. It generally affects the immunocompromised but can also infect the immunocompetent as in hot tub folliculitis. Treatment of ''P. aeruginosa'' infections can be difficult due to its natural resistance to antibiotics. When more advanced antibiotic drug regimens are needed adverse eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recombinant DNA
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome. Recombinant DNA is the general name for a piece of DNA that has been created by combining at least two fragments from two different sources. Recombinant DNA is possible because DNA molecules from all organisms share the same chemical structure, and differ only in the nucleotide sequence within that identical overall structure. Recombinant DNA molecules are sometimes called chimeric DNA, because they can be made of material from two different species, like the mythical chimera. R-DNA technology uses palindromic sequences and leads to the production of sticky and blunt ends. The DNA sequences used in the construction of recombinant DNA molecules can originate from any species. For example, plant DNA may be joined to ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Vaccine
A conjugate vaccine is a type of subunit vaccine which combines a weak antigen with a strong antigen as a carrier so that the immune system has a stronger response to the weak antigen. Vaccines are used to prevent diseases by invoking an immune response to an antigen, part of a bacterium or virus that the immune system recognizes. This is usually accomplished with an attenuated or dead version of a pathogenic bacterium or virus in the vaccine, so that the immune system can recognize the antigen later in life. Most vaccines contain a single antigen that the body will recognize. However, the antigen of some pathogens does not elicit a strong response from the immune system, so a vaccination against this weak antigen would not protect the person later in life. In this case, a conjugate vaccine is used in order to invoke an immune system response against the weak antigen. In a conjugate vaccine, the weak antigen is covalently attached to a strong antigen, thereby eliciting a stronger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GlaxoSmithKline
GSK plc, formerly GlaxoSmithKline plc, is a British Multinational corporation, multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a Mergers and acquisitions, merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tenth largest pharmaceutical company and #294 on the 2022 Fortune Global 500, ''Fortune'' Global 500, ranked behind other pharmaceutical companies China Resources, Sinopharm (company), Sinopharm, Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Bayer, and Merck Group, Merck. The company has a primary listing on the London Stock Exchange and is a constituent of the FTSE 100 Index. , it had a market capitalisation of £70 billion, the eighth largest on the London Stock Exchange. It has a secondary listing on the New York Stock Exchange. The company developed the first malaria vaccine, RTS,S, which it said in 2014 it would make available for five percent above cost. Legacy products develope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sanofi Pasteur
Sanofi Pasteur is the vaccines division of the French multinational pharmaceutical company Sanofi. Sanofi Pasteur is the largest company in the world devoted entirely to vaccines. It is one of four global producers of the yellow fever vaccine. History Since 1992, Sanofi Pasteur has sponsored Sanofi Biogenius Canada (SBC), a national, biotechnology-focused science competition for Canadian high school and CEGEP students. Those selected for the SBC work with local mentors, giving students hands-on research experience in a professional lab setting. Participants compile their results and present their findings at regional competitions. Cash prizes are awarded and regional winners advance to the National stage, where they vie for the top spot and the chance to compete in the International BioGENEius Challenge, held at the prestigious BIO International Convention – the largest biotechnology event in the world. In 2004, Aventis merged with and into Sanofi. The new Sanofi-Av ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Typhoid Vaccine
Typhoid vaccines are vaccines that prevent typhoid fever. Several types are widely available: typhoid conjugate vaccine (TCV), Ty21a (a live oral vaccine) and Vi capsular polysaccharide vaccine (ViPS) (an injectable subunit vaccine). They are about 30 to 70% effective in the first two years, depending on the specific vaccine in question. The Vi-rEPA vaccine has been shown to be efficacious in children. The World Health Organization (WHO) recommends vaccinating all children in areas where the disease is common. Otherwise they recommend vaccinating those at high risk. Vaccination campaigns can also be used to control outbreaks of disease. Depending on the vaccine, additional doses are recommended every three to seven years. In the United States the vaccine is only recommended in those at high risk such as travelers to areas of the world where the disease is common. The vaccines available as of 2018 are very safe. Minor side effects may occur at the site of injection. The injecta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |