|
Tris(triphenylphosphine)rhodium Carbonyl Hydride
Carbonyl hydrido tris(triphenylphosphine)rhodium(I) arbonyl(hydrido)tris(triphenylphosphane)rhodium(I)is an organorhodium compound with the formula hH(CO)(PPh3)3(Ph = C6H5). It is a yellow, benzene-soluble solid, which is used industrially for hydroformylation. Preparation hH(CO)(PPh3)3was first prepared by the reduction of hCl(CO)(PPh3)2 e.g. with sodium tetrahydroborate, or triethylamine and hydrogen, in ethanol in the presence of excess triphenylphosphine: : hCl(CO)(PPh3)2 + NaBH4 + PPh3 → hH(CO)(PPh3)3 + NaCl + BH3 It can also be prepared from an aldehyde, rhodium trichloride and triphenylphosphine in basic alcoholic media. Structure The complex adopts a trigonal bipyramidal geometry with trans CO and hydrido ligands, resulting in ''pseudo'' -C3v symmetry. The Rh-P, Rh-C, and Rh-H distances are 2.32, 1.83, and 1.60 Å, respectively. This complex is one of a small number of stable pentacoordinate rhodium hydrides. Use in hydroformylation This precatalyst w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organorhodium Chemistry
Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions. Stable organorhodium compounds and transient organorhodium intermediates are used as catalyst such as in olefin hydroformylation, olefin hydrogenation, olefin isomerization and the Monsanto process Classification based on principal oxidation states Organometallic rhodium compounds share many characteristics with those of iridium, but less so with cobalt. Rhodium can exist in oxidation states of -III to +V, but rhodium(I) and rhodium(III) are the more common. Rhodium(I) compounds (d8 configuration) usually occur with square planar or trigonal bipyramidal geometries, while rhodium (III) compounds (d6 configuration) typically have an octahedral geometry. Rhodium(0) Rhodium(0) complexes are binary carbonyls, the principal examples being tetrarhodium dodecacarbonyl, Rh4(CO)12, and hexadecacarbony ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociative Substitution
In chemistry, dissociative substitution describes a reaction pathway by which compounds interchange ligands. The term is typically applied to coordination and organometallic complexes, but resembles the SN1 mechanism in organic chemistry. This pathway can be well described by the ''cis'' effect, or the labilization of CO ligands in the ''cis'' position. The opposite pathway is associative substitution, being analogous to SN2 pathway. Pathways that are intermediate between the pure dissociative and pure associative pathways are called interchange mechanisms. Complexes that undergo dissociative substitution are often coordinatively saturated and often have octahedral molecular geometry. The entropy of activation is characteristically positive for these reactions, which indicates that the disorder of the reacting system increases in the rate-determining step. Kinetics Dissociative pathways are characterized by a rate determining step that involves release of a ligand from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
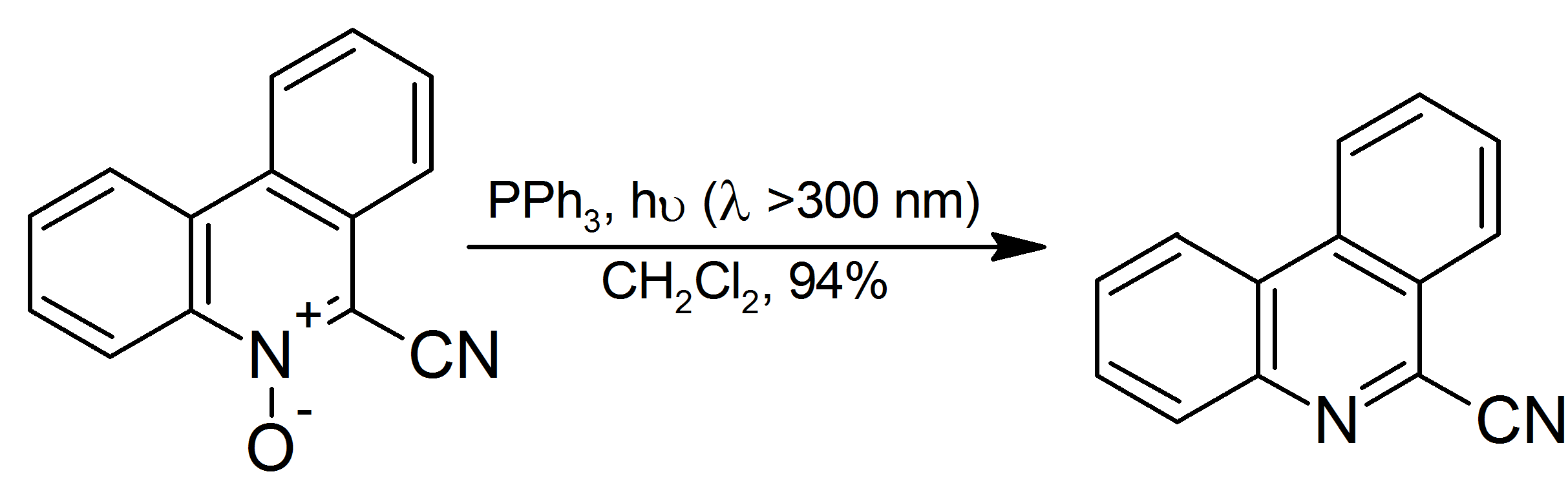
Triphenylphosphine Complexes
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups. Principal reactions with chalcogens, halogens, and acids Oxidation Triphenylphosphine undergoes slow o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalysis The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of most e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodium(I) Compounds
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope: 103Rh. Naturally occurring rhodium is usually found as a free metal or as an alloy with similar metals and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals. Rhodium is found in platinum or nickel ores with the other members of the platinum group metals. It was discovered in 1803 by William Hyde Wollaston in one such ore, and named for the rose color of one of its chlorine compounds. The element's major use (consuming about 80% of world rhodium production) is as one of the catalysts in the three-way catalytic converters in automobiles. Because rhodium metal is inert against corrosion and most aggressive chemicals, and because of its rarity, rhodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-Hydride Elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo this reaction but methyl groups cannot. The metal complex must have an empty (or vacant) site ''cis'' to the alkyl group for this reaction to occur. Moreover, for facile cleavage of the C–H bond, a d electron pair is needed for donation into the σ* orbital of the C–H bond. Thus, d0 metals alkyls are generally more stable to β-hydride elimination than d2 and higher metal alkyls and may form isolable agostic complexes, even if an empty coordination site is available. The β-hydride elimination can either be a vital step in a reaction or an unproductive side reaction. The Shell higher olefin process relies on β-hydride elimination to produce α-olefins which are used to produce detergents. Illustrative of a sometimes undesirable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Migratory Insertion
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.: : + \longrightarrow Overview In the migratory insertion, a ligand that is viewed as an anion (X) ligand in and a ligand that is viewed as neutral couple, generating a new anionic ligand. The anion and neutral ligands that react are adjacent. If the precursor complex is coordinatively saturated, migratory insertion often result in a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(triphenylphosphine)rhodium Chloride
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use. Historically, Wilkinson's catalyst has been a paradigm in catalytic studies leading to several advances in the field such as the implementation of some of the first heteronuclear magnetic resonance studies for its structural elucidation in solution (31P), parahydrogen-induced polarization spectroscopy to determine the nature of transient reactive species, or one of the first detailed kinetic investigation by Halpern to elucidate the mechanism. Furthermore, the catalytic and organome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: Production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and drugs. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry. The process entails treatment of an alkene typically with high pressures (between 10 and 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. In one variation, formaldehyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |