Organorhodium Chemistry on:
[Wikipedia]
[Google]
[Amazon]
 Organorhodium chemistry is the chemistry of
Organorhodium chemistry is the chemistry of
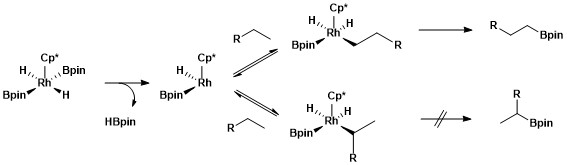
 The catalytically active species is the
The catalytically active species is the
\overset + + H2 -> ce\text] \overset

\overset + + 3CO -> ce +
 Organorhodium chemistry is the chemistry of
Organorhodium chemistry is the chemistry of organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
containing a rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring ...
-carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
, and the study of rhodium and rhodium compounds as catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s in organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical rea ...
s.
Stable organorhodium compounds and transient organorhodium intermediates are used as catalyst such as in olefin hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
, olefin hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
, olefin isomerization and the Monsanto process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chem ...
Classification based on principal oxidation states
Organometallic rhodium compounds share many characteristics with those of iridium, but less so with cobalt. Rhodium can exist inoxidation states
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
of -III to +V, but rhodium(I) and rhodium(III) are the more common. Rhodium(I) compounds (d8 configuration) usually occur with square planar or trigonal bipyramidal geometries, while rhodium (III) compounds (d6 configuration) typically have an octahedral geometry.
Rhodium(0)
Rhodium(0) complexes are binary carbonyls, the principal examples being tetrarhodium dodecacarbonyl, Rh4(CO)12, andhexadecacarbonylhexarhodium
Hexadecacarbonylhexarhodium is a metal carbonyl cluster with the formula Rh6(CO)16. It exists as purple-brown crystals that are slightly soluble in dichloromethane and chloroform. It is the principal binary carbonyl of rhodium.
Discovery and sy ...
, Rh6(CO)16. These compounds are obtained by reductive carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
of rhodium(III) salts or Rh2Cl2(CO)4. In contrast to the stability of the homologous Co2(CO)8, Rh2(CO)8 is very labile.
Rhodium(I)
Rhodium(I) complexes are importanthomogeneous catalyst
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis ...
s. Common complexes include bis(triphenylphosphine)rhodium carbonyl chloride
Bis(triphenylphosphine)rhodium carbonyl chloride is the organorhodium complex with the formula hCl(CO)(PPh3)2 This complex of rhodium(I) is a bright yellow, air-stable solid. It is the Rh analogue of Vaska's complex, the corresponding iridium ...
, chlorobis(ethylene)rhodium dimer, cyclooctadiene rhodium chloride dimer
Cyclooctadiene rhodium chloride dimer is the organorhodium compound with the formula Rh2Cl2(C8H12)2, commonly abbreviated hCl(COD)sub>2 or Rh2Cl2(COD)2. This yellow-orange, air-stable compound is a widely used precursor to homogeneous catalysts. ...
, chlorobis(cyclooctene)rhodium dimer
Chlorobis(cyclooctene)rhodium dimer is an organorhodium compound with the formula Rh2Cl2(C8H14)4, where C8H14 is ''cis''-cyclooctene. Sometimes abbreviated Rh2Cl2(coe)4, it is a red-brown, air-sensitive solid that is a precursor to many other org ...
, dicarbonyl(acetylacetonato)rhodium(I)
Dicarbonyl(acetylacetonato)rhodium(I) is an organorhodium compound with the formula Rh(O2C5H7)(CO)2. The compound consists of two CO ligands and an acetylacetonate. It is a dark green solid that dissolves in acetone and benzene, giving yellow sol ...
, and rhodium carbonyl chloride
Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful in ...
. Although not formally organometallic, Wilkinson's catalyst
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as ...
(RhCl(PPh3)3), is included in the list of important catalysts. The simple olefin complexes chlorobis(ethylene)rhodium dimer, chlorobis(cyclooctene)rhodium dimer, and cyclooctadiene rhodium chloride dimer are often used as sources of "RhCl", exploiting the lability of the alkene ligands or their susceptibility to removal by hydrogenation. (η5- Cp)RhL2 are derived from Rh2Cl2L4 (L = CO, C2H4).
Rhodium(II)
Unlike the prevalence of cobalt(II) complexes, compounds of rhodium(II) are rare. Thesandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic de ...
rhodocene
Rhodocene is a chemical compound with the formula . Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic ...
is one example, even it exists in equilibrium with a dimeric Rh(I) derivative. Although not organometallic, rhodium(II) acetate
Rhodium(II) acetate is the coordination compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for cyclopropanation of al ...
(Rh2(OAc)4) catalyzes cyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane () rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolon ...
s via organometallic intermediates. Rhodium(II) porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical ...
complexes react with methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
.
Rhodium(III)
Rhodium is usually supplied commercially in the Rh(III) oxidation state, the main starting reagent being hydratedrhodium trichloride
Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)''n'', where ''n'' varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of ''n'', the material is either a den ...
. The latter reacts with olefins and with CO to give organometallic complexes, often concomitant with reduction to Rh(I). Cyclopentadienyl complexes of rhodium include the half-sandwich compound
Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples in ...
pentamethylcyclopentadienyl rhodium dichloride dimer
Pentamethylcyclopentadienyl rhodium dichloride dimer is an organometallic compound with the formula C5(CH3)5RhCl2)sub>2, commonly abbreviated p*RhCl2sub>2 This dark red air-stable diamagnetic solid is a reagent in organometallic chemistry.
St ...
.
Rhodium(V)
Strong donor ligands - hydride, silyl, boryl - are required to stabilize Rh(V). This oxidation state is invoked inborylation Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen ...
reactions.

Metallacycles
Cyclometalatedrhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring ...
compounds constitute an important class of organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
chemistry. Although such compounds are well documented in the literature rhodium(III) cyclometalates with azo function are spare. A typical example of this category viz. novel hexacoordinated orthometalated rhodium(III) thiolato complex trans- h(C∧N∧S)Cl(PPh3)2was synthesized from benzyl 2-(phenylazo)phenyl thioether and RhCl3·3H2O in the presence of excess PPh3 via in situ C(sp2)−H and C(sp3)−S bond scissions. This is the first example for a coordination compound of (phenylazo)thiolate ligand. The mechanism of formation of orthometalated azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of simi ...
derivative was described to proceed via initial coordination of azo-nitrogen followed by electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that car ...
substitution at the pendant phenyl ring. PPh3 plays a crucial role in the C(sp3)−S cleavage process. Reductive cleavage by single electron transfer
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spon ...
(SET) mechanism is likely to be operative for the C−S bond cleavage. Unlike analogous (phenylazo)phenolato compound the orthometalated thiolato complex exhibits a fully reversible oxidative wave at 0.82 V vs Ag/AgCl and this response is supposed to be primarily centered on the thiolato sulfur atom.
Main applications
Despite its high cost, rhodium is heavily relied on as a commercial catalyst.Acetic acid and acetic anhydride syntheses
TheMonsanto process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chem ...
is an industrial method for the making of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
by catalytic carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
of methanol, although it has largely been supplanted by the iridium-based Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an ...
.
anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
''cis''- h(CO)2I2sup>−. which undergoes oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxid ...
with methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one ...
. The related Tennessee Eastman acetic anhydride process affords acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a c ...
by carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
of methyl acetate
Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish remo ...
.
: CH3CO2CH3 + CO → (CH3CO)2O
Hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
s often rely on rhodium-based catalysts. Water-soluble catalysts have also been developed. They facilitate the separation of the products from the catalyst.
:Hydrogenation
Wilkinson's catalyst is used as ahomogeneous catalyst
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis ...
for the hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
of olefins. The mechanism of catalysis involves oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxid ...
of H2, π-complexation of alkene, migratory insertion
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mecha ...
(intramolecular hydride transfer or olefin insertion), and reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and ...
.
Cationic organorhodium(I) catalysts are useful for asymmetric hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from othe ...
s, which are applied to bioactive products such as pharmaceutical agents
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field an ...
and agrochemicals
An agrochemical or agrichemical, a contraction of ''agricultural chemical'', is a chemical product used in industrial agriculture. Agrichemical refers to biocides (pesticides including insecticides, herbicides, fungicides and nematicides) an ...
.

Other reactions
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor ...
reduction is another reaction catalysed by this compound type:
:References
{{ChemicalBondsToCarbon