|
Tricarbon
Tricarbon (systematically named 1λ2,3λ2-propadiene and ''catena''-tricarbon) is an inorganic compound with the chemical formula (also written (μ-C)Cor ). It is a colourless gas that only persists in dilution or solution as an adduct. It is one of the simplest unsaturated carbenes. Tricarbon can be found in interstellar space and can be produced in the laboratory by a process called laser ablation. Natural occurrence Tricarbon is a small carbon cluster first spectroscopically observed in the early 20th century in the tail of a comet by William Huggins and subsequently identified in stellar atmospheres. Small carbon clusters like tricarbon and dicarbon are regarded as soot precursors and are implicated in the formation of certain industrial diamonds and in the formation of fullerenes. C3 has also been identified as a transient species in various combustion reactions. Properties Chemical properties The chemical properties of C3 was investigated in the 1960s by Prof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Molecules In Interstellar Space
This is a list of molecules that have been detected in the interstellar medium and circumstellar envelopes, grouped by the number of component atoms. The chemical formula is listed for each detected compound, along with any ionized form that has also been observed. Background The molecules listed below were detected through astronomical spectroscopy. Their spectral features arise because molecules either absorb or emit a photon of light when they transition between two molecular energy levels. The energy (and thus the wavelength) of the photon matches the energy difference between the levels involved. Molecular electronic transitions occur when one of the molecule's electrons moves between molecular orbitals, producing a spectral line in the ultraviolet, visible light, optical or near-infrared parts of the electromagnetic spectrum. Alternatively, a vibrational transition transfers Quantum, quanta of energy to (or from) vibrations of molecular bonds, producing signatures in the mid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philip Skell
Philip S. Skell (December 30, 1918 – November 21, 2010) was an American chemist, emeritus Evan Pugh Professor at Pennsylvania State University, and from 1977 a member of the United States National Academy of Sciences. Biography Skell was born in Brooklyn, New York, to Jacob and Molly Lipfriend Skell. He married Margo Fosse on December 25, 1948, in Taft. He held a Bachelor of Science degree from City College of New York, a master's degree from Columbia University and a doctorate from Duke University. During World War II, at the National Center for Agricultural Utilization Research and as a post-doc at the University of Illinois, Skell took part in the early work on the production of Penicillin. Research At Penn State, Skell's field of research were then hypothetical very short-lived Reaction intermediates like free radicals, Carbonium ions, Tricarbon and Carbene, whose existence and properties he could demonstrate by use of Chemical traps. Applying new experimental techniques he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
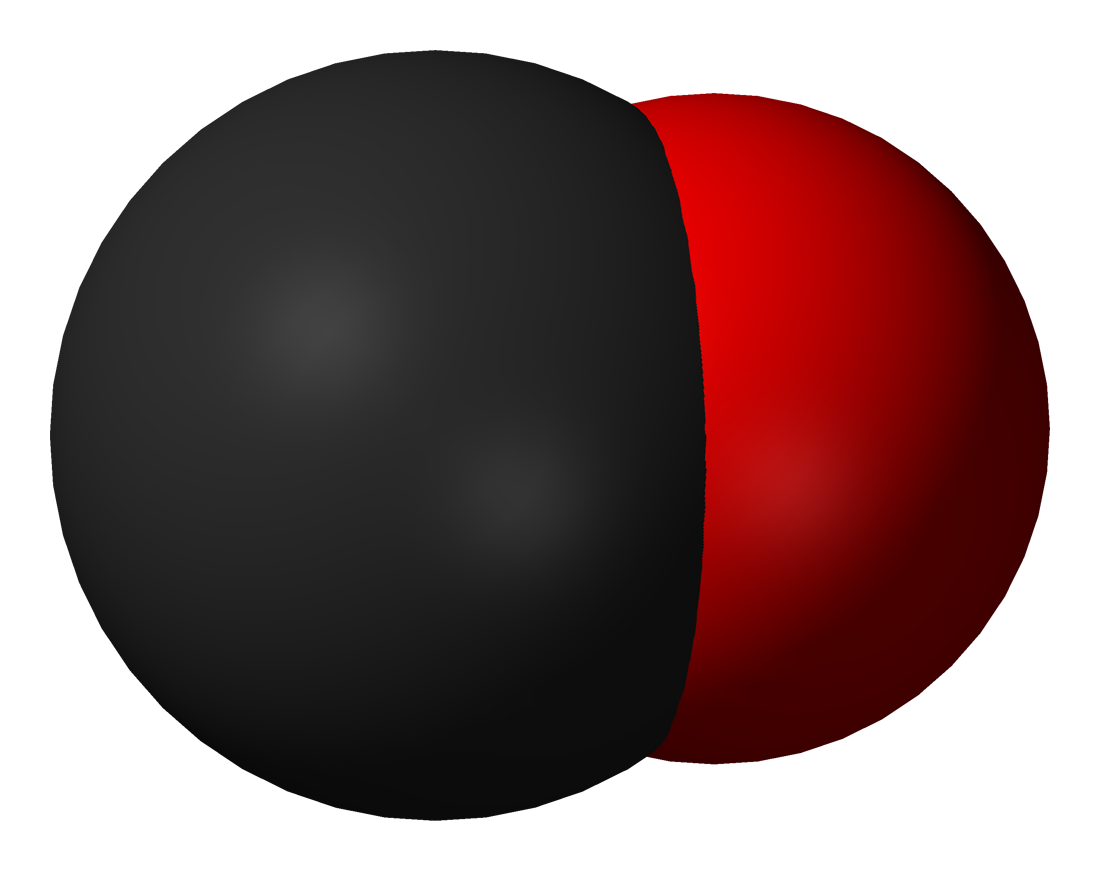
Diatomic Carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and in blue hydrocarbon flames. Diatomic carbon is the second simplest form of carbon after atomic carbon, and is an intermediate participator in the genesis of fullerenes. Properties C2 is a component of carbon vapor. One paper estimates that carbon vapor is around 28% diatomic, but theoretically this depends on the temperature and pressure. Electromagnetic properties The electrons in diatomic carbon are distributed among the molecular orbitals according to the Aufbau principle to produce unique quantum states, with corresponding energy levels. The state with the lowest energy level, or ground s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can give molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropatriene
Cyclopropatriene is a hypothetical compound () which is an allotrope of carbon. It was once proposed as a candidate for a spectroscopically observed tricarbon species. It is a cyclic cumulene In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also .... References Allotropes of carbon Hypothetical chemical compounds Three-membered rings Homonuclear triatomic molecules {{theoretical-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propadiene
Propadiene () or allene () is the organic compound with the formula . It is the simplest allene, i.e. a compound with two adjacent carbon double bonds. As a constituent of MAPP gas, it has been used as a fuel for specialized welding. Production and equilibrium with methylacetylene Allene exists in equilibrium with methylacetylene (propyne) and the mixture is sometimes called MAPD for ''m''ethyl''a''cetylene-''p''ropa''d''iene: :H3CC#CH <<=> H2C=C=CH2 for which at 270 °C or 0.1 at 5 °C. MAPD is produced as a side product, often an undesirable one, of of to produce , an important |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira (ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/MEDLINE/PubMed, and the Science Citation Index Expanded. According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879–1880 – Hermann Endemann * 1880–1881 – Gideon E. Moore * 1881–1882 – Hermann Endemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy In physics, the kinetic energy of an object is the energy that it possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acc ... gained by a single electron accelerating from rest through an Voltage, electric potential difference of one volt in vacuum. When used as a Units of energy, unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the Electric charge, charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in Particle accelerator#Electrostatic particle accelerators, electrostatic particle accel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |