|
List Of Molecules In Interstellar Space
This is a list of molecules that have been detected in the interstellar medium and circumstellar envelopes, grouped by the number of component atoms. The chemical formula is listed for each detected compound, along with any ionized form that has also been observed. Background The molecules listed below were detected through astronomical spectroscopy. Their spectral features arise because molecules either absorb or emit a photon of light when they transition between two molecular energy levels. The energy (and thus the wavelength) of the photon matches the energy difference between the levels involved. Molecular electronic transitions occur when one of the molecule's electrons moves between molecular orbitals, producing a spectral line in the ultraviolet, optical or near-infrared parts of the electromagnetic spectrum. Alternatively, a vibrational transition transfers quanta of energy to (or from) vibrations of molecular bonds, producing signatures in the mid- or far-infrared. G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vibrational Transition
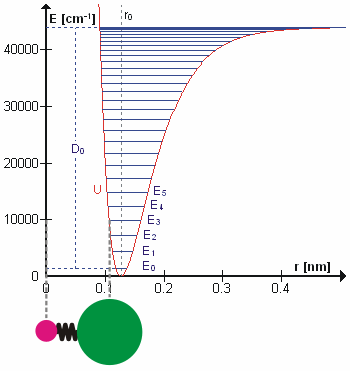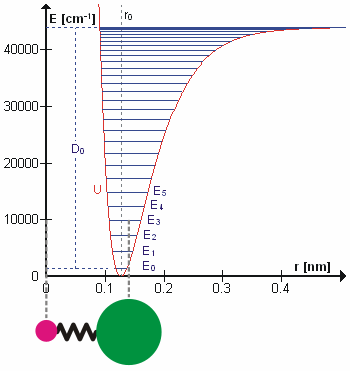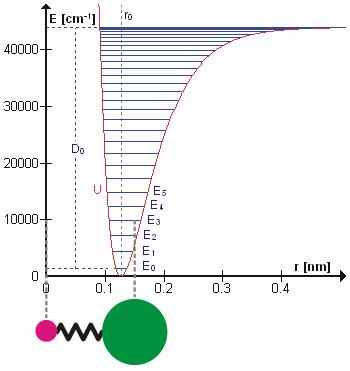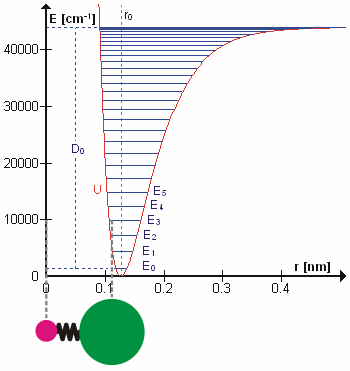
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 Hz, corresponding to wavenumbers of approximately 300 to 3000 cm−1 and wavelengths of approximately 30 to 3 µm. For a diatomic molecule A−B, the vibrational frequency in s−1 is given by \nu = \frac \sqrt , where k is the force constant in dyne/cm or erg/cm2 and μ is the reduced mass given by \frac = \frac+\frac. The vibrational wavenumber in cm−1 is \tilde \;= \frac \sqrt, where c is the speed of light in cm/s. Vibrations of polyatomic molecules are described in terms of normal modes, which are independent of each other, but each normal mode involves simultaneous vibrations of different parts of the molecule. In general, a non-linear molecule with ''N'' atoms has 3''N'' – 6 normal modes of vibration, but a ''linea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions to physical and chemical properties of molecules, materials, and solutions at the atomic level. These calculations include systematically applied approximations intended to make calculations computationally feasible while still capturing as much information about important contributions to the computed wave functions as well as to observable properties such as structures, spectra, and thermodynamic properties. Quantum chemistry is also concerned with the computation of quantum effects on molecular dynamics and chemical kinetics. Chemists rely heavily on spectroscopy through which information regarding the quantization of energy on a molecular scale can be obtained. Common methods are infra-red (IR) spectroscopy, nuclear magnetic r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selection Rules
In physics and chemistry, a selection rule, or transition rule, formally constrains the possible transitions of a system from one quantum state to another. Selection rules have been derived for electromagnetic transitions in molecules, in atoms, in atomic nuclei, and so on. The selection rules may differ according to the technique used to observe the transition. The selection rule also plays a role in chemical reactions, where some are formally spin-forbidden reactions, that is, reactions where the spin state changes at least once from reactants to products. In the following, mainly atomic and molecular transitions are considered. Overview In quantum mechanics the basis for a spectroscopic selection rule is the value of the ''transition moment integral'' :\int \psi_1^* \, \mu \, \psi_2 \, \mathrm\tau\,, where \psi_1 and \psi_2 are the wave functions of the two states, "state 1" and "state 2", involved in the transition, and is the transition moment operator. Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotational Spectroscopy
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of chemical polarity, polar molecules can be measured in Absorption (optics), absorption or Emission (electromagnetic radiation), emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as ''pure'' rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously. For rotational spectroscopy, molecules are classified according to symmetry into spherical top, linear and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vibronic Band
Vibronic spectroscopy is a branch of molecular spectroscopy concerned with vibronic transitions: the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the appropriate energy. In the gas phase, vibronic transitions are accompanied by changes in rotational energy also. Vibronic spectra of diatomic molecules have been analysed in detail; emission spectra are more complicated than absorption spectra. The intensity of allowed vibronic transitions is governed by the Franck–Condon principle. Vibronic spectroscopy may provide information, such as bond length, on electronic excited states of stable molecules. It has also been applied to the study of unstable molecules such as dicarbon, C2, in discharges, flames and astronomical objects.Hollas, p. 211. Principles Electronic transitions are typically observed in the visible and ultraviolet regions, in the wavelength range approximately 200–700&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diatomic Carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and in blue hydrocarbon flames. Diatomic carbon is the second simplest form of carbon after atomic carbon, and is an intermediate participator in the genesis of fullerenes. Properties C2 is a component of carbon vapor. One paper estimates that carbon vapor is around 28% diatomic, but theoretically this depends on the temperature and pressure. Electromagnetic properties The electrons in diatomic carbon are distributed among the molecular orbitals according to the Aufbau principle to produce unique quantum states, with corresponding energy levels. The state with the lowest energy level, or ground ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radio
Radio is the technology of signaling and communicating using radio waves. Radio waves are electromagnetic waves of frequency between 30 hertz (Hz) and 300 gigahertz (GHz). They are generated by an electronic device called a transmitter connected to an antenna which radiates the waves, and received by another antenna connected to a radio receiver. Radio is very widely used in modern technology, in radio communication, radar, radio navigation, remote control, remote sensing, and other applications. In radio communication, used in radio and television broadcasting, cell phones, two-way radios, wireless networking, and satellite communication, among numerous other uses, radio waves are used to carry information across space from a transmitter to a receiver, by modulating the radio signal (impressing an information signal on the radio wave by varying some aspect of the wave) in the transmitter. In radar, used to locate and track objects like aircraft, ships, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequency, frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ranges as microwaves; the above broad definition includes both Ultra high frequency, UHF and Extremely high frequency, EHF (millimeter wave) bands. A more common definition in radio-frequency engineering is the range between 1 and 100 GHz (wavelengths between 0.3 m and 3 mm). In all cases, microwaves include the entire Super high frequency, SHF band (3 to 30 GHz, or 10 to 1 cm) at minimum. Frequencies in the microwave range are often referred to by their Radio band#IEEE, IEEE radar band designations: S band, S, C band (IEEE), C, X band, X, Ku band, Ku, K band (IEEE), K, or Ka band, Ka band, or by similar NATO or EU designations. The prefix ' in ''microwave'' is not meant to suggest a wavelength in the M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
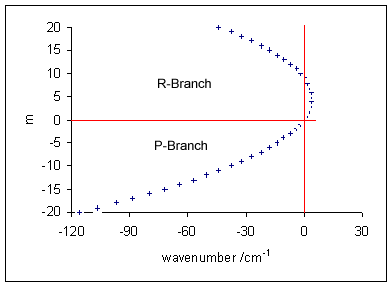
Rotational Transition
In quantum mechanics, a rotational transition is an abrupt change in angular momentum. Like all other properties of a quantum particle, angular momentum is quantized, meaning it can only equal certain discrete values, which correspond to different rotational energy states. When a particle loses angular momentum, it is said to have transitioned to a lower rotational energy state. Likewise, when a particle gains angular momentum, a positive rotational transition is said to have occurred. Rotational transitions are important in physics due to the unique spectral lines that result. Because there is a net gain or loss of energy during a transition, electromagnetic radiation of a particular frequency must be absorbed or emitted. This forms spectral lines at that frequency which can be detected with a spectrometer, as in rotational spectroscopy or Raman spectroscopy. Diatomic molecules Molecules have rotational energy owing to rotational motion of the nuclei about their center of ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |