|
Thorium Dioxide
Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is produced mainly as a by-product of lanthanide and uranium production. Thorianite is the name of the mineralogical form of thorium dioxide. It is moderately rare and crystallizes in an isometric system. The melting point of thorium oxide is 3300 °C – the highest of all known oxides. Only a few elements (including tungsten and carbon) and a few compounds (including tantalum carbide) have higher melting points. All thorium compounds, including the dioxide, are radioactive because there are no stable isotopes of thorium. Structure and reactions Thoria exists as two polymorphs. One has a fluorite crystal structure. This is uncommon among binary dioxides. (Other binary oxides with fluorite structure include cerium dioxide, uranium dioxide and plutonium dioxide.) The band gap of thoria is about 6 eV. A tetragonal form of thoria is also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word "alkali" is derived from Arabic ''al qalīy'' (or ''alkali''), meaning ''the calcined ashes'' (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
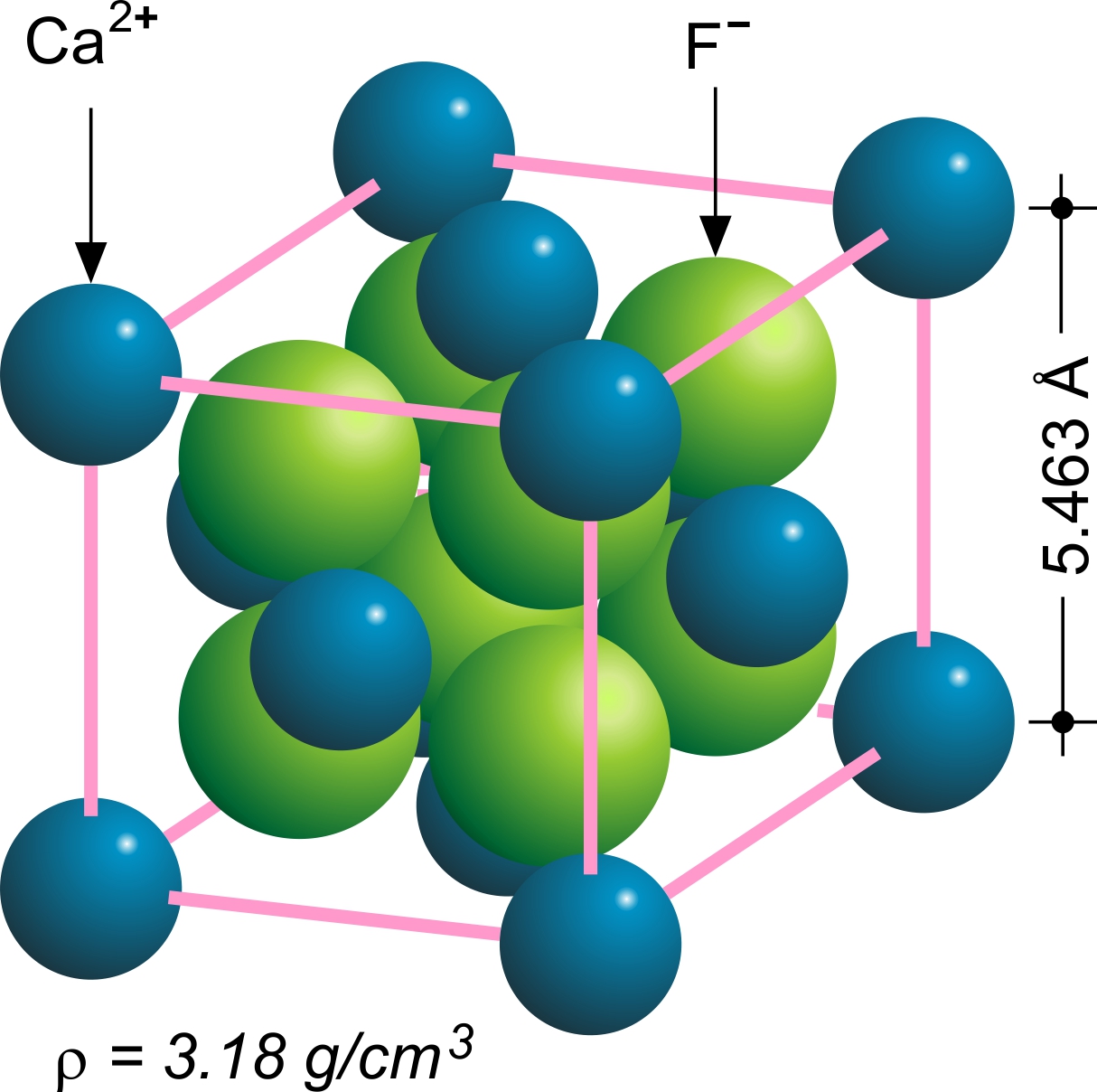
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Inert Gas Welding
Gas tungsten arc welding (GTAW), also known as tungsten inert gas (TIG) welding, is an arc welding process that uses a non-consumable tungsten electrode to produce the weld. The weld area and electrode are protected from oxidation or other atmospheric contamination by an inert shielding gas (argon or helium). A filler metal is normally used, though some welds, known as ''autogenous welds'', or ''fusion welds'' do not require it. When helium is used, this is known as heliarc welding. A constant-current welding power supply produces electrical energy, which is conducted across the arc through a column of highly ionized gas and metal vapors known as a plasma. GTAW is most commonly used to weld thin sections of stainless steel and non-ferrous metals such as aluminum, magnesium, and copper alloys. The process grants the operator greater control over the weld than competing processes such as shielded metal arc welding and gas metal arc welding, allowing for stronger, higher quality w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Stability
In thermodynamics, thermal stability describes the stability of a water body and its resistance to mixing.Schmidt, W. 1928. Über Temperatur und Stabilitätsverhältnisse von Seen. Geogr. Ann 10: 145 - 177. It is the amount of work needed to transform the water to a uniform water density. The Schmidt stability "S" is commonly measured in joule The joule ( , ; symbol: J) is the unit of energy in the International System of Units (SI). It is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 metre in the direction of the force applied ...s per square meter (J/m). References Further reading * Molecular physics {{engineering-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle and they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction. In principle, a reaction can involve more than two particles collision, colliding, but because the probability of three or more nuclei to meet at the same time at the same place is much less than for two nuclei, such an event is exceptionally rare (see triple alpha process for an example very close to a three-body nuclear reaction). The term "nuclear reaction" may refer either to a change in a nuclide induced by collision with another particle or to a spontaneous change of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium-233
Uranium-233 (233U or U-233) is a fissile Isotopes of uranium, isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a Nuclear fuel, reactor fuel. It has been used successfully in experimental nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years. Uranium-233 is produced by the neutron irradiation of thorium-232. When thorium-232 absorbs a neutron, it becomes thorium-233, which has a half-life of only 22 minutes. Thorium-233 decays into protactinium-233 through beta decay. Protactinium-233 has a half-life of 27 days and beta decays into uranium-233; some proposed molten salt reactor designs attempt to physically isolate the protactinium from further neutron capture before beta decay can occur, to maintain the neutron economy (if it misses the 233U window, the next fissile target is 235U, meaning a total of 4 neutrons nee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation Reaction
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type, regardless of whether it is a redox or some other type of process: :2A -> A' + A'' Examples *Mercury(I) chloride disproportionates upon UV-irradiation: :Hg2Cl2 → Hg + HgCl2 *Phosphorous acid disproportionates upon heating to give phosphoric acid and phosphine: :4 → 3 H3PO4 + PH3 *Desymmetrizing reactions are sometimes referred to as disproportionation, as illustrated by the thermal degradation of bicarbonate: :2 → + H2CO3 :The oxidation numbers remain constant in this acid-base reaction. This process is also called autoionization. *Another variant on disproportionation is radical disproportionation, in which two radicals form an alkene and an alkane. : Reverse r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium Monoxide
Thorium monoxide (thorium(II) oxide), is the binary oxide of thorium having chemical formula ThO. The covalent bond in this diatomic molecule is highly polar. The effective electric between the two atoms has been calculated to be about 80 gigavolts per centimeter, one of the largest known internal effective electric fields. Simple combustion of thorium in air produces thorium dioxide. However, laser ablation Laser ablation or photoablation (also called laser blasting) is the process of removing material from a solid (or occasionally liquid) surface by irradiating it with a laser beam. At low laser flux, the material is heated by the absorbed laser ... of thorium in the presence of oxygen gives the monoxide. Additionally, exposure of a thin film of thorium to low-pressure oxygen at medium temperature forms a rapidly growing layer of thorium monoxide under a more-stable surface coating of the dioxide. At extremely high temperatures, thorium dioxide can convert to the mono ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy In physics, the kinetic energy of an object is the energy that it possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acc ... gained by a single electron accelerating from rest through an Voltage, electric potential difference of one volt in vacuum. When used as a Units of energy, unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the Electric charge, charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in Particle accelerator#Electrostatic particle accelerators, electrostatic particle accel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium Dioxide
Plutonium(IV) oxide or (plutonia) is the chemical compound with the formula Pu O2. This high melting-point solid is a principal compound of plutonium. It can vary in color from yellow to olive green, depending on the particle size, temperature and method of production. Structure PuO2 crystallizes in the fluorite motif, with the Pu4+ centers organized in a face-centered cubic array and oxide ions occupying tetrahedral holes. PuO2 owes its utility as a nuclear fuel to the fact that vacancies in the octahedral holes allows room for fission products. In nuclear fission, one atom of plutonium splits into two. The vacancy of the octahedral holes provides room for the new product and allows the PuO2 monolith to retain its structural integrity. Properties Plutonium dioxide is a stable ceramic material with an extremely low solubility in water and with a high melting point (2,744 °C). The melting point was revised upwards in 2011 by several hundred degrees, based on evidence from r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |