|
Tellurol
Tellurols are analogues of alcohols and phenols where tellurium replaces oxygen. Tellurols, selenols, and thiols have similar properties, but tellurols are the least stable. Although they are fundamental representatives of organotellurium compounds, tellurols are lightly studied because of their instability. Tellurol derivatives include telluroesters (RC(O)TeR') and tellurocyanates (RTeCN). Properties Alkyltellurols are colorless liquids with strong odors. Samples usually appear yellowish owing to the presence of dialkylditelluride impurities. Near room temperature, methanetellurol degrades with loss of elemental tellurium. It is reported to ignite in air. Aryltellurols are more robust and have been obtained as colorless crystals. Some of the most stable tellurols are the bulky silylated derivatives of tris(trimethylsilyl)methane and analogues. One series of readily isolable tellurols is , , and . Acid–base properties The acidity of tellurols can be inferred by the acidity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanetellurol
Methanetellurol is the organotellurium compound with the formula CH3TeH. It is the simplest organotellurium compound that has been purified in bulk. It is classified as a tellurol. A colorless gas, it decomposes to Te and methane near room temperature. It is prepared by reduction of dimethyl ditelluride using Na/NH3 followed by protonation of the NaTeCH3 with sulfuric acid. Few publications describe this compound as a consequence of its instability and paucity of applications. According to IR spectroscopy, νTe-H = 1995 cm−1. For the lighter homologues, νE-H = 2342 (E = Se), 2606 (E = S), and 3710 cm−1 (E = O) for methaneselenol, methanethiol Methanethiol (also known as methyl mercaptan) is an organosulfur compound with the chemical formula . It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals (including humans ..., and methanol.{{cite journal , doi=10.1080/00945717708069709, title=The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.Anderson, Don L.; "Chemical Composition of the Mantle" in ''Theory of the Earth'', pp. 147-175 Tellurium-bearing compounds were first discovered in 1782 in a gold mine in Kleinschlatten, Transylvania (now Zlatna, Romania) by Austrian mineralogist Franz-Joseph Müller von Reichenstein, although it was Martin Heinrich Klaproth who named the new element in 1798 after the Latin 'earth'. Gold telluride minerals ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(trimethylsilyl)methane
Tris(trimethylsilyl)methane is the organosilicon compound with the formula (tms)3CH (where tms = (CH3)3Si). It is a colorless liquid that is highly soluble in hydrocarbon solvents. Reaction of tris(trimethylsilyl)methane with methyl lithium gives tris(trimethylsilyl)methyllithium, called trisyllithium. Trisyllithium is useful in Petersen olefination reactions: :(tms)3CH + CH3Li → (tms)3CLi + CH4 :(tms)3CLi + R2CO → (tms)2C=CR2 + tmsOLi Trisyllithium is also an effective precursor to bulky ligands. Some tris(trimethylsilyl)methyl derivatives are far more stable than less substituted derivatives. for example is a well-behaved tellurol Tellurols are analogues of alcohols and phenols where tellurium replaces oxygen. Tellurols, selenols, and thiols have similar properties, but tellurols are the least stable. Although they are fundamental representatives of organotellurium compound .... See also * Tris(trimethylsilyl)silane References Carbosilanes Trim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Telluride
Hydrogen telluride is the inorganic compound with the formula hydrogen, H2telluride (chemistry), Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist at very low concentrations long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Synthesis Electrolytic methods have been developed.F. Fehér, "Hydrogen Telluride" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Russian Chemical Reviews
The ''Russian Chemical Reviews'' is a translation of ''Journal Uspekhi Khimii'' which is a monthly Russian scientific journal on chemistry, established in 1932. The journal cover aspects of modern chemistry. According to the ''Journal Citation Reports'', its 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... is 6.926 References Monthly journals Russian Academy of Sciences academic journals {{chemistry-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
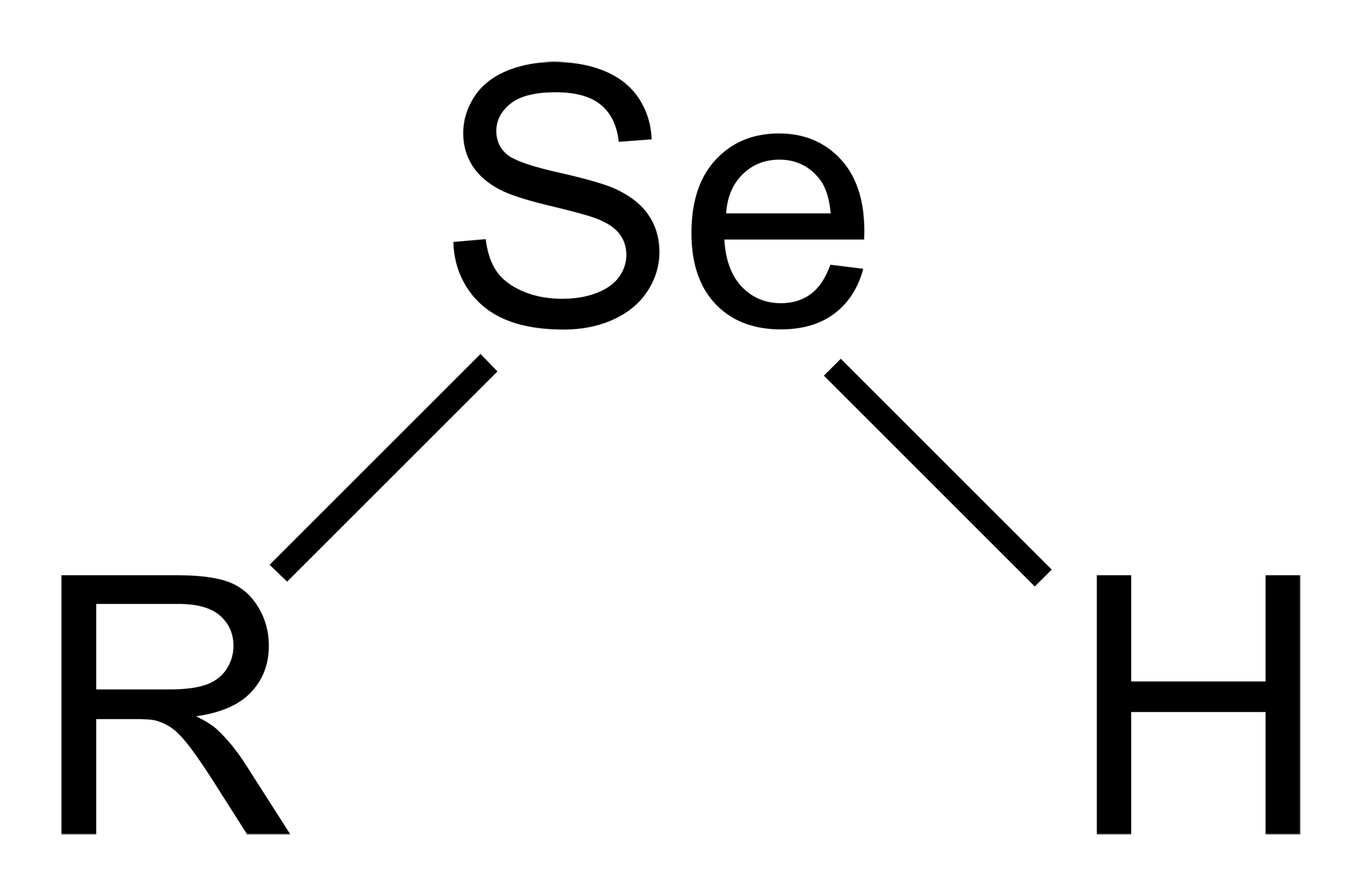
Selenol
Selenols are organic compounds that contain the functional group with the connectivity C–Selenium, Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member of the group is the amino acid selenocysteine. Structure, bonding, properties Selenols are structurally similar to thiols, but the C-Se bond is about 8% longer at 196 pm. The C–Se–H angle approaches 90°. The bonding involves almost pure p-orbitals on Se, hence the near 90 angles. The Se–H bond energy is weaker than the S–H bond, consequently selenols are easily oxidized and serve as H-atom donors. The Se-H bond is much weaker than the S-H bond as reflected in their respective bond dissociation energy (BDE). For C6H5Se-H, the BDE is 326 kJ/mol, while for C6H5S-H, the BDE is 368 kJ/mol. Selenol acids are about 1000 times stronger than thiols: the p''K''a of CH3SeH is 5.2 vs 8.3 for CH3SH. Deprotonation affo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry (journal)
''Inorganic Chemistry'' is a biweekly peer-reviewed scientific journal published by the American Chemical Society since 1962. It covers research in all areas of inorganic chemistry. The current editor-in-chief is William B. Tolman (Washington University). Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 5.436. See also * ''Organometallics ''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is organometallic and organometalloid chemistry. This peer-reviewed journal has an impact factor of 3.837 as reported by the 2021 Journal Cit ...'' References External links * American Chemical Society academic journals Biweekly journals Publications established in 1962 English-language journals Inorganic chemistry journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotellurium Compound
Organotellurium chemistry describes the synthesis and properties of chemical compounds containing a carbon-tellurium chemical bond. Organotellurium chemistry is a lightly studied area, in part because of the few applications. Functional groups The Te analogues of common organosulfur and organoselenium functional groups are known. Tellurols are however unstable with respect to oxidation to the ditellurides. Commonly encountered organotellurium compounds are diorganomono- and ditellurides, R2Te and (RTe)2, respectively. Two other families of organoTe(IV) compounds are well developed: R4−xTeClx and the telluroxides (R2TeO). Synthesis and reactions Reduced organoTe compounds Reduced organoTe compounds are commonly obtained from NaHTe and lithium telluride: :Li2Te + 2 RBr → R2Te + 2 LiBr A direct route to organolithium compounds starts from reactions of organolithium or Grignard reagents and Te: :Te + ArLi → ArTeLi Butyl lithium gives the telluride similarly: :Te + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |