Selenol on:
[Wikipedia]
[Google]
[Amazon]
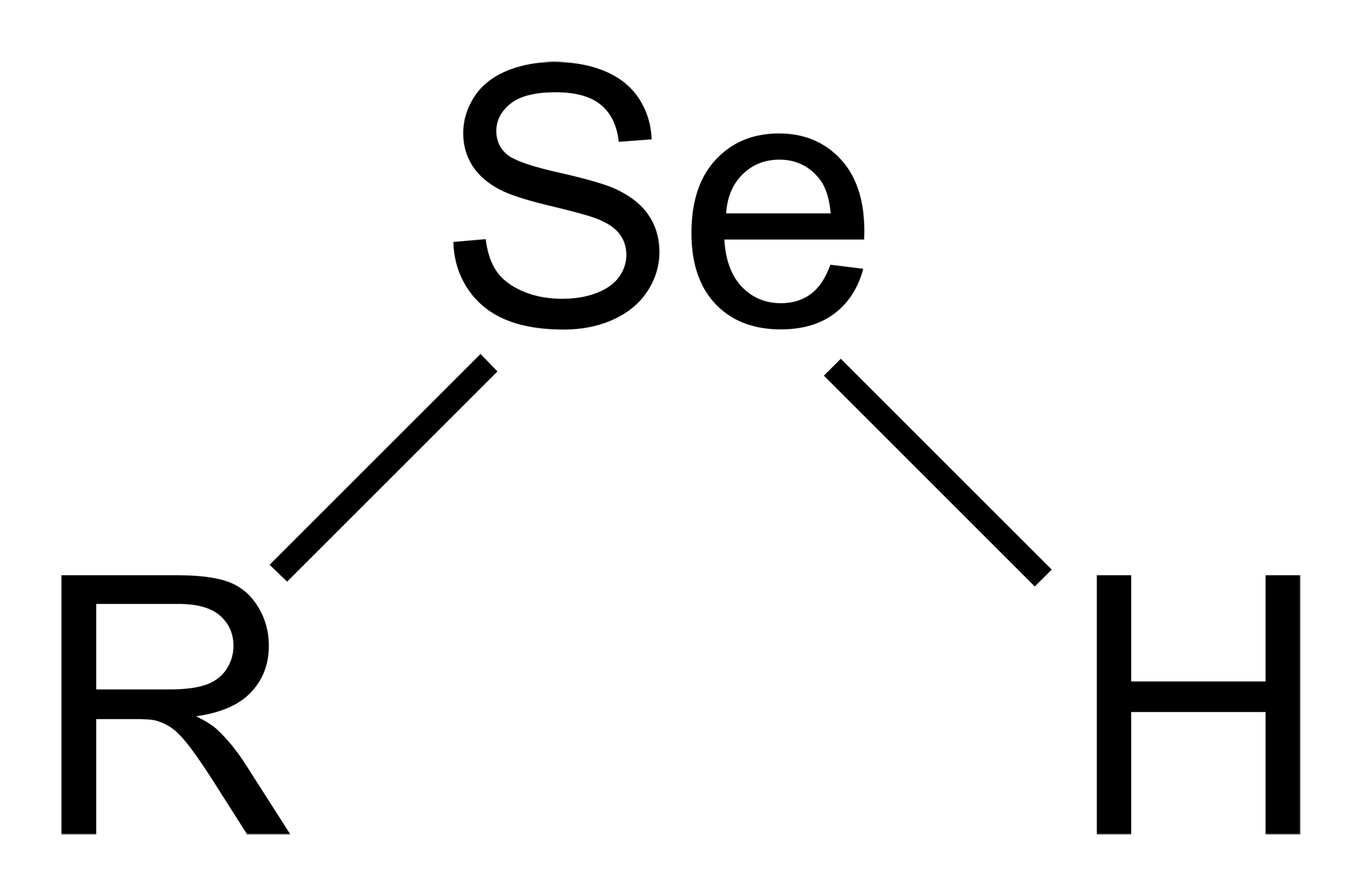 Selenols are
Selenols are
 Another preparative route to selenols involves the
Another preparative route to selenols involves the
 Selenols are
Selenols are organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s that contain the functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
with the connectivity C– Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compound
Organoselenium compounds (or seleno-organic) are chemical compounds containing carbon-to- selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and su ...
s. The best known member of the group is the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
selenocysteine
Selenocysteine (symbol Sec or U, in older publications also as Se-Cys) is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the ...
.
Structure, bonding, properties
Selenols are structurally similar tothiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
s, but the C-Se bond is about 8% longer at 196 pm. The C–Se–H angle approaches 90°. The bonding involves almost pure p-orbitals on Se, hence the near 90 angles. The Se–H bond energy
In chemistry, bond energy (''BE''), also called the mean bond enthalpy or average bond enthalpy is the measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually ...
is weaker than the S–H bond, consequently selenols are easily oxidized and serve as H-atom donors. The Se-H bond is much weaker than the S-H bond as reflected in their respective bond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical ...
(BDE). For C6H5Se-H, the BDE is 326 kJ/mol, while for C6H5S-H, the BDE is 368 kJ/mol.
Selenol acids are about 1000 times stronger than thiols: the p''K''a of CH3SeH is 5.2 vs 8.3 for CH3SH. Deprotonation affords the selenolate anion, RSe−, most examples of which are highly nucleophilic and rapidly oxidized by air.
The boiling points of selenols tend to be slightly greater than for thiols. This can be attributed to the increased importance of stronger van der Waals bond
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
ing for larger atoms. Volatile selenols have highly offensive odors.
Applications and occurrence
Selenols have few commercial applications, being limited by the high toxicity of selenium as well as the sensitivity of the Se–H bond. Theirconjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
s, the selenolates, do have limited applications in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
.
Biochemical role
Selenols are important in certain biological processes. Three enzymes found in mammals contain selenols at their active sites:glutathione peroxidase
Glutathione peroxidase (GPx) () is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid h ...
, iodothyronine deiodinase
Iodothyronine deiodinases ( and ) are a subfamily of deiodinase enzymes important in the activation and deactivation of thyroid hormones. Thyroxine (T4), the precursor of 3,5,3'-triiodothyronine (T3) is transformed into T3 by deiodinase activity ...
, and thioredoxin reductase
Thioredoxin reductases (TR, TrxR) () are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. In bacteria TrxR also catalyzes the reduction ...
. The selenols in these proteins are part of the essential amino acid
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life form ...
selenocysteine
Selenocysteine (symbol Sec or U, in older publications also as Se-Cys) is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the ...
. The selenols function as reducing agents to give selenenic acid
A selenenic acid is an organoselenium compound and an oxoacid with the general formula RSeOH, where R ≠ H. It is the first member of the family of organoselenium oxoacids, which also include seleninic acids and selenonic acids, which are RSeO ...
derivative (RSe–OH), which in turn are re-reduced by thiol-containing enzymes. Methaneselenol (commonly named "methylselenol") (CH3SeH), which can be produced in vitro by incubating selenomethionine
Selenomethionine (SeMet) is a naturally occurring amino acid. The L-selenomethionine enantiomer is the main form of selenium found in Brazil nuts, cereal grains, soybeans, and grassland legumes, while ''Se''-methylselenocysteine, or its γ-gluta ...
with a bacterial methionine gamma-lyase (METase) enzyme, by biological methylation of selenide ion or in vivo by reduction of methaneseleninic acid (CH3SeO2H), has been invoked to explain the anticancer activity of certain organoselenium compounds. Precursors of methaneselenol are under active investigation in cancer prevention and therapy. In these studies, methaneselenol is found to be more biologically active than ethaneselenol or 2-propaneselenol.
Preparation
Selenols are usually prepared by the reaction oforganolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s or Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
s with elemental Se. For example, benzeneselenol
Benzeneselenol, also known as selenophenol, is the organoselenium compound with the formula C6H5SeH, often abbreviated PhSeH. It is the selenium analog of phenol. This colourless, malodorous compound is a reagent in organic synthesis.
Synthesis
...
is generated by the reaction of phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is ...
with selenium followed by acidicifation:
: Another preparative route to selenols involves the
Another preparative route to selenols involves the alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
of selenourea
Selenourea is the organoselenium compound with the formula SeC(NH2)2. It is a white solid. This compound features a rare example of a stable, unhindered carbon-selenium double bond. The compound is used in the synthesis of selenium heterocycles ...
, followed by hydrolysis. Selenols are often generated by reduction of diselenides followed by protonation of the resulting selenoate:
:2 RSeSeR + 2 LiHB(C2H5)3 → 2 RSeLi + 2 B(C2H5)3 + H2
:RSeLi + HCl → RSeH + LiCl
Dimethyl diselenide can be easily reduced to methaneselenol within cells.
Reactions
Selenols are easily oxidized to diselenides, compounds containing an Se-Se bond. For example, treatment of benzeneselenol with bromine givesdiphenyl diselenide
Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This orange-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis.
Preparation a ...
.
:2 C6H5SeH + Br2 → (C6H5Se)2 + 2 HBr
In the presence of base, selenols are readily alkylated to give selenides. This relationship is illustrated by the methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These ...
of methaneselenol to give dimethylselenide
Dimethyl selenide is the organoselenium compound with the formula (CH3)2Se. This colorless, malodorous, liquid is the simplest selenoether. It occurs in trace amounts in anaerobic environments.
Dimethyl selenide is prepared by treating Se2- sour ...
.
Safety
Organoselenium compound
Organoselenium compounds (or seleno-organic) are chemical compounds containing carbon-to- selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and su ...
s (or any selenium compound) are cumulative poisons despite the fact that trace amounts of Se are required for health.
See also
*Alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
* Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
References
{{Functional group Functional groups Selenols