|
Tris(trimethylsilyl)methane
Tris(trimethylsilyl)methane is the organosilicon compound with the formula (tms)3CH (where tms = (CH3)3Si). It is a colorless liquid that is highly soluble in hydrocarbon solvents. Reaction of tris(trimethylsilyl)methane with methyl lithium gives tris(trimethylsilyl)methyllithium, called trisyllithium. Trisyllithium is useful in Petersen olefination reactions: :(tms)3CH + CH3Li → (tms)3CLi + CH4 :(tms)3CLi + R2CO → (tms)2C=CR2 + tmsOLi Trisyllithium is also an effective precursor to bulky ligands. Some tris(trimethylsilyl)methyl derivatives are far more stable than less substituted derivatives. for example is a well-behaved tellurol Tellurols are analogues of alcohols and phenols where tellurium replaces oxygen. Tellurols, selenols, and thiols have similar properties, but tellurols are the least stable. Although they are fundamental representatives of organotellurium compound .... See also * Tris(trimethylsilyl)silane References Carbosilanes Trim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosilicon Compound
Organosilicon compounds are organometallic compounds containing carbon–silicon chemical bond, bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic chemistry, inorganic'' compound. History In 1846 Von Ebelman's had synthesized Tetraethyl orthosilicate (Si(OC2H5)4). In 1863 Friedel and Crafts managed to make the first organosilieon compound with C-Si bonds which gone byound the syntheses of orthosilicic acid esters. The same year they also described a «polysilicic acid ether» in the preparation of Ethanol, ethyl- and methyl-o-silicic acid. The early extensive research in the field of organosilicon compounds was pioneerd in the beginning of 20th century by Frederic Kipping. He also had coined the term «silicone» (akin to ketones) in relation to these materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications. A trimethylsilyl group bonded to a methyl group forms tetramethylsilane, which is abbreviated as TMS as well. Compounds with trimethylsilyl groups are not normally found in nature. Chemists sometimes use a trimethylsilylating reagent to derivatize rather non-volatile compounds such as certain alcohols, phenols, or carboxylic acids by substituting a trimethylsilyl group for a hydrogen in the hydroxyl groups on the compounds. This way trimethylsiloxy groups minus;O-Si(CH3)3are formed on the molecule. A couple of examples of trimethylsilylating agents include trimethylsilyl chloride and bis(trimethylsilyl)acetamide. Trime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
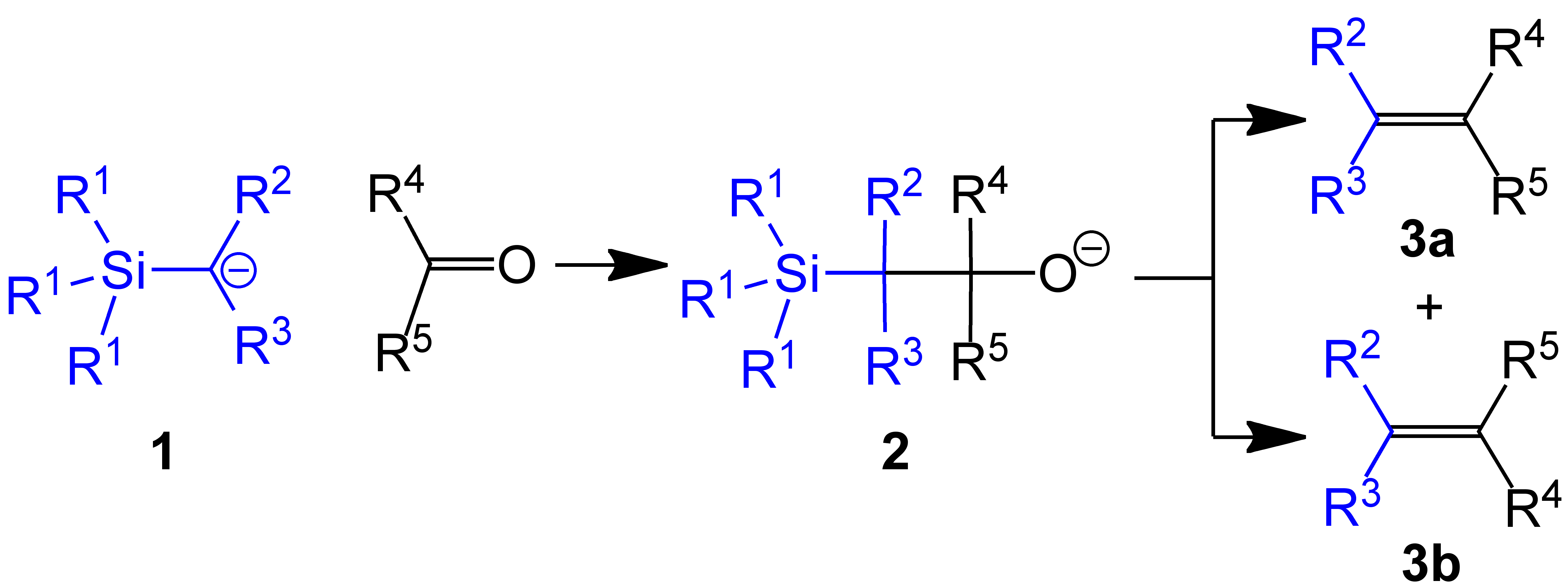
Petersen Olefination
The Peterson olefination (also called the Peterson reaction) is the chemical reaction of α-silyl carbanions (1 in diagram below) with ketones (or aldehydes) to form a β-hydroxysilane (2) which eliminates to form alkenes (3). Several reviews have been published.Ager, D. J. ''Org. React.'' 1990, ''38'', 1. Reaction mechanism One attractive feature of the Peterson olefination is that it can be used to prepare either cis- or trans-alkenes from the same β-hydroxysilane. Treatment of the β-hydroxysilane with acid will yield one alkene, while treatment of the same β-hydroxysilane with base will yield the alkene of opposite stereochemistry. Basic elimination The action of base upon a β-hydroxysilane (1) results in a concerted ''syn'' elimination of (2) or (3) to form the desired alkene. The penta-coordinate silicate intermediate (3) is postulated, but no proof exists to date. Potassium alkoxides eliminate quickly, while sodium alkoxides generally require heating. Magnes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurol
Tellurols are analogues of alcohols and phenols where tellurium replaces oxygen. Tellurols, selenols, and thiols have similar properties, but tellurols are the least stable. Although they are fundamental representatives of organotellurium compounds, tellurols are lightly studied because of their instability. Tellurol derivatives include telluroesters (RC(O)TeR') and tellurocyanates (RTeCN). Properties Alkyltellurols are colorless liquids with strong odors. Samples usually appear yellowish owing to the presence of dialkylditelluride impurities. Near room temperature, methanetellurol degrades with loss of elemental tellurium. It is reported to ignite in air. Aryltellurols are more robust and have been obtained as colorless crystals. Some of the most stable tellurols are the bulky silylated derivatives of tris(trimethylsilyl)methane and analogues. One series of readily isolable tellurols is , , and . Acid–base properties The acidity of tellurols can be inferred by the acidity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Russian Chemical Reviews
The ''Russian Chemical Reviews'' is a translation of ''Journal Uspekhi Khimii'' which is a monthly Russian scientific journal on chemistry, established in 1932. The journal cover aspects of modern chemistry. According to the ''Journal Citation Reports'', its 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... is 6.926 References Monthly journals Russian Academy of Sciences academic journals {{chemistry-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(trimethylsilyl)silane
Tris(trimethylsilyl)silane is the organosilicon compound with the formula (Me3Si)3SiH (where Me = CH3). It is a colorless liquid that is classified as a hydrosilane since it contains an Si-H bond. The compound is notable as having a weak Si-H bond, with a bond dissociation energy estimated at 84 kcal/mol. For comparison, the Si-H bond in trimethylsilane is 94 kcal/mol. With such a weak bond, the compound is used as a reagent to deliver hydrogen atoms. The compound has been described as an environmentally benign analogue of tributyltin hydride Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. Synthesis and characterization The .... The compound can be prepared by protonation of tris(trimethylsilyl)silyl lithium, which is derived from tetrakis(trimethylsilyl)silane: :(Me3Si)4Si + MeLi → (Me3Si)3SiLi + Me4Si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbosilanes
Carbosilanes are organosilicon compounds where the structures feature alternating silicon and carbon atoms, i.e., Si-C-Si-C linkages. They represent molecular analogues of silicon carbide. The compounds exploit the tendency of both carbon and silicon to form tetrahedral structures. The inventory of carbosilanes is large.{{cite book , doi=10.1007/978-3-642-70800-8, title=Carbosilanes , year=1986 , last1=Fritz , first1=Gerhard , last2=Matern , first2=Eberhard , isbn=978-3-642-70802-2 Synthesis and structure The compounds originally were obtained as products of the pyrolysis The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ... of simple organosilicon precursors such as the methylsilanes. More efficient precursors contain premade Si-C-Si-C etc. subunits. References Carbosilanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-3D-balls.png)
