|
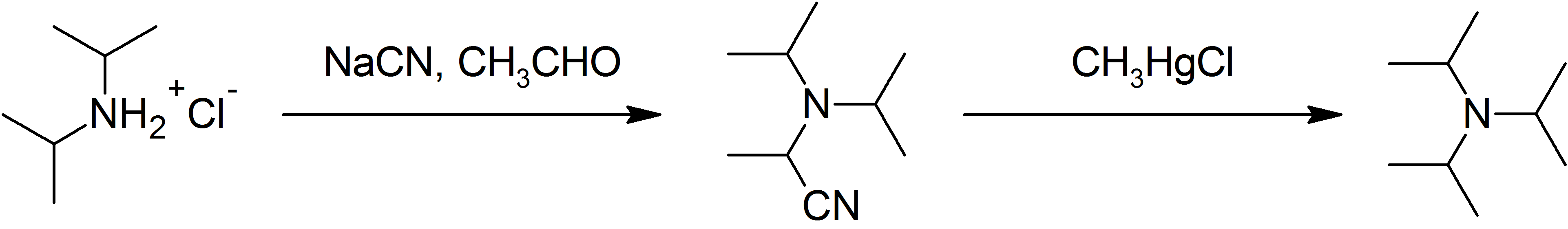
Triisopropylamine
Triisopropylamine is an organic chemical compound consisting of three isopropyl groups bound to a central nitrogen atom. As a hindered tertiary amine, it can be used as a non-nucleophilic base and as a stabilizer for polymers; however, its applications are limited by its relatively high cost and difficult synthesis. Structure Triisopropylamine is notable as being among the most sterically hindered amines currently known. The even more crowded tri-''tert''-butylamine (''t''Bu3N) has never been synthesized, although ''ab initio'' quantum chemical computations as well as the existence of the even more crowded 2,2,4,4-tetramethyl-3-''t''-butyl-pentane-3-ol (tri-''tert''-butylcarbinol, ''t''Bu3COH) implies that it should be a stable molecule if it could be prepared. To date, di-''tert''-butyl(isopropyl)amine (''t''Bu2iPrN) has been prepared in low yield, as have a handful of tri-''tert''-alkylamines in which two of the ''tert''-alkyl groups are tied together in a ring, but the auth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylenetriamine
Diethylenetriamine (abbreviated and also known as 2,2’-Iminodi(ethylamine)) is an organic compound with the formula HN(CH2CH2NH2)2. This colourless hygroscopic liquid is soluble in water and polar organic solvents, but not simple hydrocarbons. Diethylenetriamine is structural analogue of diethylene glycol. Its chemical properties resemble those for ethylene diamine, and it has similar uses. It is a weak base and its aqueous solution is alkaline. DETA is a byproduct of the production of ethylenediamine from ethylene dichloride. Reactions and uses Diethylenetriamine is a common curing agent for epoxy resins in epoxy adhesives and other thermosets. It is N-alkylated upon reaction with epoxide groups forming crosslinks. In coordination chemistry, it serves as a tridentate ligand forming complexes such as Co(dien)(NO2)3. Like some related amines, it is used in oil industry for the extraction of acid gas. Like ethylenediamine, DETA can also be used to sensitize nitromethane, ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a colorless, hygroscopic, and flammable tertiary amine. It is a gas at room temperature but is usually sold as a 40% solution in water. (It is also sold in pressurized gas cylinders.) TMA is a nitrogenous base and can be readily protonated to give the trimethylammonium cation. Trimethylammonium chloride is a hygroscopic colorless solid prepared from hydrochloric acid. Trimethylamine is a good nucleophile, and this reaction is the basis of most of its applications. TMA is widely used in industry: it is used in the synthesis of choline, tetramethylammonium hydroxide, plant growth regulators or herbicides, strongly basic anion exchange resins, dye leveling agents, and a number of basic dyes. At higher concentrations it has an ammonia-like odor, and can cause necrosis of mucous membranes on contact. At lower concentrations, it has a "fishy" odor, the odor associated with rotting fish. In humans, ingesti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HN3 (nitrogen Mustard)
Tris(2-chloroethyl)amine is the organic compound with the formula N(CH2CH2Cl)3. Often abbreviated HN3 or HN-3, it is a powerful blister agent and a nitrogen mustard used for chemical warfare. HN3 was the last of the nitrogen mustard agents developed. It was designed as a military agent and is the only one of the nitrogen mustards that is still used for military purposes. It is the principal representative of the nitrogen mustards because its vesicant properties are almost equal to those of HD and thus the analogy between the two types of mustard is the strongest.NITROGEN MUSTARD HN-3 Emergency Response Safety and Health Database. |
Tris(2-aminoethyl)amine
Tris(2-aminoethyl)amine is the organic compound with the chemical formula, formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Abbreviated tren or TREN it is a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry. Tren is a C3-symmetric, tetradentate chelating ligand that forms stable complexes with transition metals, especially those in the 2+ and 3+ oxidation states. Tren complexes exist with relatively few isomers, reflecting the constrained connectivity of this tetramine. Thus, only a single achiral stereoisomer exists for [Co(tren)X2]+, where X is halide or pseudohalide. In contrast, for [Co(trien)X2]+ five diastereomers are possible, four of which are chiral. In a few cases, tren serves as a tridentate ligand with one of the primary amine groups non-coordinated. Tren is a common impurity in the more common trie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N,N-Diisopropylethylamine
''N'',''N''-Diisopropylethylamine, or Hünig's base, is an organic compound and an amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a base. It is commonly abbreviated as DIPEA, DIEA, or ''i''-Pr2NEt. Structure DIPEA consists of a central nitrogen atom that is bonded to an ethyl group and two isopropyl groups. A lone pair of electrons resides on the nitrogen atom, which can react with electrophiles. However, as the two isopropyl groups and the ethyl group occupy much of the space surrounding the N atom, only small electrophiles such as protons can react with the nitrogen lone pair. Occurrence and preparation DIPEA is commercially available. It is traditionally prepared by the alkylation of diisopropylamine with diethyl sulfate. Pure DIPEA exists as a colorless liquid, although commercial samples can be slightly yellow. If necessary, the compound can be purified by distillation from potassium hydroxide or calcium hydride. Use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechlorethamine
Chlormethine (INN, BAN), also known as mechlorethamine (USAN, USP), mustine, HN2, and (in post-Soviet states) embikhin (эмбихин), is a nitrogen mustard sold under the brand name Mustargen among others. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance. Mechlorethamine belongs to the group of nitrogen mustard alkylating agents. Uses It has been derivatized into the estrogen analogue estramustine phosphate, used to treat prostate cancer. It can also be used in chemical warfare where it has the code-name HN2. This chemical is a form of nitrogen mustard gas and a powerful vesicant. Historically, some uses of mechlorethamine have included lymphoid ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylaminopropylamine
Dimethylaminopropylamine (DMAPA) is a diamine used in the preparation of some surfactants, such as cocamidopropyl betaine which is an ingredient in many personal care products including soaps, shampoos, and cosmetics. BASF, a major producer, claims that DMAPA-derivatives do not sting the eyes and makes a fine-bubble foam, making it appropriate in shampoos. Preparation and reactions DMAPA is commonly produced commercially via the reaction between dimethylamine and acrylonitrile (a Michael reaction) to produce dimethylaminopropionitrile. A subsequent hydrogenation step yields DMAPA: : DMAPA is readily converted to the mustard dimethylaminopropyl-3-chloride, a powerful alkylating agent. Health effects Dimethylaminopropylamine is a known skin irritant and its presence as an impurity in cocamidopropyl betaine is thought to be the cause of irritation experienced by some individuals. See also * 1,1-Dimethylethylenediamine * 1,2-Dimethylethylenediamine References {{reflist Diamine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisopropylamine
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent. Reactions and use Diisopropylamine is a common amine nucleophile in organic synthesis. Because it is bulky, it is a more selective nucleophile than, say, dimethylamine. It reacts with organolithium reagents to give lithium diisopropylamide (LDA). LDA is a strong, non-nucleophilic base The main commercial applications of diisopropylamine is as a precursor to two herbicides, diallate and triallate, as well as certain sulfenamides used in the vulcanization of rubber. It is also used to prepare N,N-Diisopropylethylamine (Hünig's base) by alkylation with diethyl sulfate. The bromide salt of diisopropylamine, diisopropylammonium bromide, is a room-temperature organic ferroelectric material. Preparation Diisopropylamine, which is comme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The |
Trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a colorless, hygroscopic, and flammable tertiary amine. It is a gas at room temperature but is usually sold as a 40% solution in water. (It is also sold in pressurized gas cylinders.) TMA is a nitrogenous base and can be readily protonated to give the trimethylammonium cation. Trimethylammonium chloride is a hygroscopic colorless solid prepared from hydrochloric acid. Trimethylamine is a good nucleophile, and this reaction is the basis of most of its applications. TMA is widely used in industry: it is used in the synthesis of choline, tetramethylammonium hydroxide, plant growth regulators or herbicides, strongly basic anion exchange resins, dye leveling agents, and a number of basic dyes. At higher concentrations it has an ammonia-like odor, and can cause necrosis of mucous membranes on contact. At lower concentrations, it has a "fishy" odor, the odor associated with rotting fish. In humans, ingesti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |