|
Trifluorooxonium
The trifluorooxonium cation is a hypothetical positively charged polyatomic ion with chemical formula . It is structurally equivalent to the hydronium ion where the hydrogen atoms surrounding the central oxygen atom have been replaced by fluorine, and is isoelectronicity, isoelectronic with nitrogen trifluoride. This cation would be an example of oxygen in the unprecedented +4 oxidation state. The cation was shown to be vibrationally stable at all levels of theory applied (HF, MP2, CCSD(T)). was proposed to possess a pyramidal structure with an O–F bond length of 1.395 Å and F–O–F bond angles of 104.2° (CCSD(T) level of theory). The detachment energy of the cation was calculated to be +110.1 kcal mol−1. However, the low-temperature reaction of , and under UV irradiation, besides unreacted starting materials only yielded the dioxygenyl salt . The oxidation of with salts also failed to produce evidence for the title cation. The formation of the hypothetica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, ) to the surrounding water molecules (). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous and conjugate base. Three main structures for the aqueous proton have garnered experimental support: The Eigen cation, which is a tetrahydrate, H3O+(H2O)3; the Zundel cation, which is a symmetric dihydrate, H+(H2O)2; and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroammonium
The tetrafluoroammonium cation (also known as perfluoroammonium) is a positively charged polyatomic ion with chemical formula . It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane , trifluoramine oxide and the tetrafluoroborate anion. The tetrafluoroammonium ion forms salts with a large variety of fluorine-bearing anions. These include the bifluoride anion (), tetrafluorobromate (), metal pentafluorides ( where M is Ge, Sn, or Ti), hexafluorides ( where M is P, As, Sb, Bi, or Pt), heptafluorides ( where M is W, U, or Xe), octafluorides (), various oxyfluorides ( where M is W or U; , ), and perchlorate (). Attempts to make the nitrate salt, , were unsuccessful because of quick fluorination: + → + . Structure The geometry of the tetrafluoroammonium ion is tetrahedral, with an estimated nitrogen-fluorine bond length of 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoelectronicity
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the structure. For example, , , and are isoelectronic, while and = are not. This definition is sometimes termed ''valence isoelectronicity''. Definitions can sometimes be not as strict, sometimes requiring identity of the ''total'' electron count and with it the entire electronic configuration. More usually, definitions are broader, and may extend to allowing different numbers of atoms in the species being compared.A. A. Aradi & T. P. Fehlner, "Isoelectronic Organometallic Molecules", in F. G. A. Stone & Robert West (eds.) ''Advances in Organometallic Chemistry Vol. 30'' (1990), Chapter 5 (at p. 190google books link/ref> The importance of the concept lies in identifying significantly related species, as pairs or series. Isoelectronic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
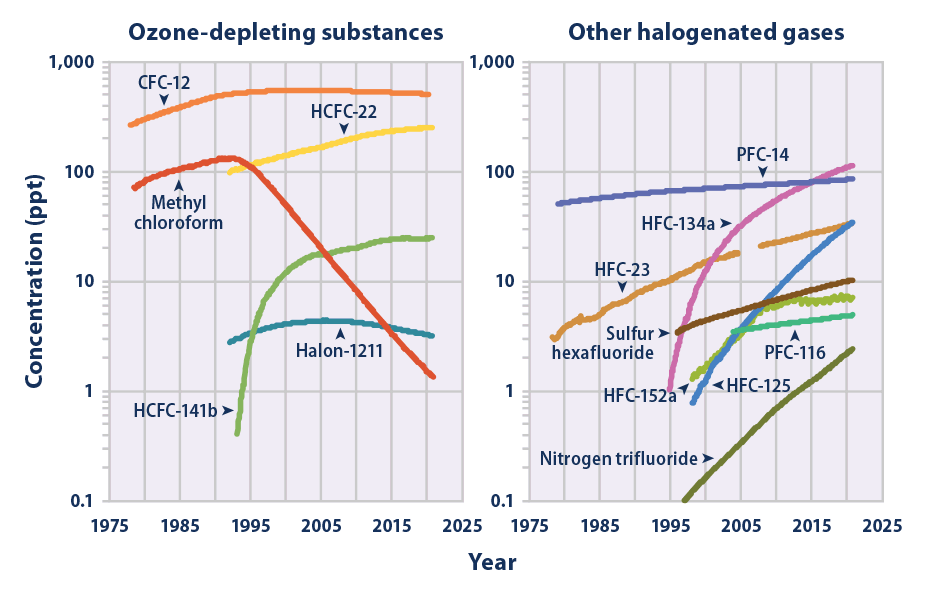
Nitrogen Trifluoride
Nitrogen trifluoride () is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century. Synthesis and reactivity Nitrogen trifluoride did not exist in significant quantities on Earth prior to its synthesis by humans. It is a rare example of a binary fluoride that can be prepared directly from the elements only at very uncommon conditions, such as an electric discharge. After first attempting the synthesis in 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride. It proved to be far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen trib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxygenyl
The dioxygenyl ion, , is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of . It is formally derived from oxygen by the removal of an electron: :O2 → + e− The energy change for this process is called the ionization energy of the oxygen molecule. Relative to most molecules, this ionization energy is very high at 1175 kJ/mol. As a result, the scope of the chemistry of is quite limited, acting mainly as a 1-electron oxidiser. Structure and molecular properties has a bond order of 2.5, and a bond length of 112.3 pm in solid O2 sF6 It is isoelectronic with nitric oxide and is paramagnetic. The bond energy is 625.1 kJ mol−1 and the stretching frequency is 1858 cm−1, both of which are high relative to most of the molecules. Synthesis Neil Bartlett demonstrated that dioxygenyl hexafluoroplatinate (O2PtF6), containing the dioxygenyl cation, can be prepared at room temperature by direct reaction of oxygen gas (O2) with platinum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxonium Compounds in England
{{Disambiguation ...
Oxonium may refer to: *Oxonium ion, any ion which contains a trivalent oxygen atom, *Oxonium, an IUPAC name for the simplest oxonium ion, hydronium, *''Oxonium'' (often abbreviated to ''Oxon.''), sometimes used in university circles as a Latin name for Oxford University Oxford () is a city in England. It is the county town and only city of Oxfordshire. In 2020, its population was estimated at 151,584. It is north-west of London, south-east of Birmingham and north-east of Bristol. The city is home to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |