|
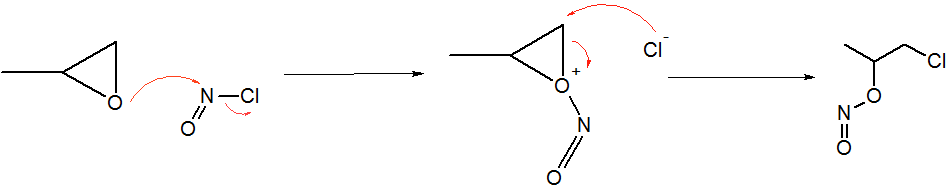
Trichloronitrosomethane
Trichloronitrosomethane is a chlorinated nitrosoalkane. It is a deep blue liquid with powerful lachrymatory effects. Synthesis Trichloronitrosomethane can be produced with following methods: * Oxidation of trichloromethylsulfinic acid with nitric acid. * Reaction of sodium trichloromethylsulfinate with sodium nitrite and sodium nitrate or potassium nitrate in sulfuric acid. * Pyrolysis of trichloroacethydroxamic acid. Chemistry Trichloronitrosomethane is an unstable substance. It slowly decomposes into nitrosyl chloride, nitrogen oxides, and chloropicrin over time. Trichloronitrosomethane can be reduced to phosgene oxime by hydrogen sulfide. See also *Chloropicrin * Trifluoronitrosomethane *Phosgene oxime Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disag ... References Nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloropicrin
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. It was used as a poison gas in World War I. Its chemical structural formula is Cl3CNO2. Synthesis Chloropicrin was discovered in 1848 by Scottish chemist John Stenhouse. He prepared it by the reaction of sodium hypochlorite with picric acid: : HOC6H2(NO2)3 + 11 NaOCl → 3 Cl3CNO2 + 3 Na2CO3 + 3 NaOH + 2 NaCl Because of the precursor used, Stenhouse named the compound chloropicrin, although the two compounds are structurally dissimilar. Today, chloropicrin is manufactured by the reaction of nitromethane with sodium hypochlorite: : H3CNO2 + 3 NaOCl → Cl3CNO2 + 3 NaOH or by the reaction of chloroform with nitric acid: : CHCl3 + HNO3 → CCl3NO2 + H2O Properties Chloropicrin's chemical formula is CCl3NO2 and its molecular weight is 164.38 grams/mole. Pure chloropicrin is a colorless liquid, with a boiling po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoronitrosomethane
Trifluoronitrosomethane (commonly abbreviated TFNM) is a toxic organic compound consisting of a trifluoromethyl group covalently bound to a nitroso group. Its distinctive deep blue color is unusual for a gas. History Trifluoronitrosomethane was synthesized for the first time in 1936 by Otto Ruff and Manfred Giese at the University of Wrocław. It was created through the fluorination of silver cyanide in the presence of silver nitrate and silver oxide. Production Trifluoronitrosomethane can be produced from the reaction of trifluoroiodomethane and nitric oxide under a UV light with a yield of up to 90% in normal pressure. A small amount of mercury is needed as catalyst. The reaction results in the creation of iodine as a by-product. Properties Although it is somewhat more kinetically stable due to its fluorine substituents, trifluoronitrosomethane, like other nitroso compounds, has a weak C–N bond of only 39.9 kcal/mol. Related Trifluoronitrosoethylene is also a similar d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloropicrin
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. It was used as a poison gas in World War I. Its chemical structural formula is Cl3CNO2. Synthesis Chloropicrin was discovered in 1848 by Scottish chemist John Stenhouse. He prepared it by the reaction of sodium hypochlorite with picric acid: : HOC6H2(NO2)3 + 11 NaOCl → 3 Cl3CNO2 + 3 Na2CO3 + 3 NaOH + 2 NaCl Because of the precursor used, Stenhouse named the compound chloropicrin, although the two compounds are structurally dissimilar. Today, chloropicrin is manufactured by the reaction of nitromethane with sodium hypochlorite: : H3CNO2 + 3 NaOCl → Cl3CNO2 + 3 NaOH or by the reaction of chloroform with nitric acid: : CHCl3 + HNO3 → CCl3NO2 + H2O Properties Chloropicrin's chemical formula is CCl3NO2 and its molecular weight is 164.38 grams/mole. Pure chloropicrin is a colorless liquid, with a boiling po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloromethyl Compounds
The trichloromethyl group is a functional group that has the formula –CCl3. The naming of is group is derived from the methyl group (which has the formula –CH3), by replacing each hydrogen atom by a chlorine atom. Compounds with this group are a subclass of the organochlorines. Some notable examples of compounds with this group are trichloromethane H–, 1,1,1-trichloroethane –, and chloral –. The trichloromethyl group has a significant electronegativity. For this reason, trichloromethyl-substituted acids, such as trichloromethanesulfonic acid, are often stronger than the original. For example, the acidity constant (pKa) of trichloroacetic acid – is 0.77, whereas that of acetic acid is 4.76. By the same principle, the trichloromethyl group generally lowers the basicity of organic compounds, e.g. trichloroethanol 2,2,2-Trichloroethanol is the chemical compound with formula . Its molecule can be described as that of ethanol, with the three hydrogen atoms at position ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroso Compounds
In organic chemistry, nitroso refers to a functional group in which the nitric oxide () group is attached to an organic moiety. As such, various nitroso groups can be categorized as ''C''-nitroso compounds (e.g., nitrosoalkanes; ), ''S''-nitroso compounds ( nitrosothiols; ), ''N''-nitroso compounds (e.g., nitrosamines, ), and ''O''-nitroso compounds ( alkyl nitrites; ). Synthesis Nitroso compounds can be prepared by the reduction of nitro compounds or by the oxidation of hydroxylamines. Ortho-nitrosophenols may be produced by the Baudisch reaction. In the Fischer–Hepp rearrangement aromatic 4-nitrosoanilines are prepared from the corresponding nitrosamines. Properties Nitrosoarenes typically participate in a monomer–dimer equilibrium. The dimers, which are often pale yellow, are often favored in the solid state, whereas the deep-green monomers are favored in dilute solution or at higher temperatures. They exist as ''cis'' and ''trans'' isomers. Due to the stability o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosgene Oxime
Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disagreeable odor and a violently irritating vapor. It is seldom used but is a precursor of compounds with fungicidal, biocidal and pesticide activity. Preparation and reactions Phosgene oxime can be prepared by reduction of chloropicrin using a combination of tin metal and hydrochloric acid as the source of the active hydrogen reducing acent: : The observation of a transient violet color in the reaction suggests intermediate formation of trichloronitrosomethane (Cl3CNO). Early preparations, using stannous chloride as the reductant, also started with chloropicrin. The compound is electrophilic and thus sensitive to nucleophiles, including bases: : Phosgene oxime has been used to prepare heterocycles that contain N-O bonds, such as isoxaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is ''stinkdamp''. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. The British English spelling of this compound is hydrogen sulphide, a spelling no longer recommended by the Royal Society of Chemistry or the International Union of Pure and Applied Chemistry. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or it or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosgene Oxime
Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disagreeable odor and a violently irritating vapor. It is seldom used but is a precursor of compounds with fungicidal, biocidal and pesticide activity. Preparation and reactions Phosgene oxime can be prepared by reduction of chloropicrin using a combination of tin metal and hydrochloric acid as the source of the active hydrogen reducing acent: : The observation of a transient violet color in the reaction suggests intermediate formation of trichloronitrosomethane (Cl3CNO). Early preparations, using stannous chloride as the reductant, also started with chloropicrin. The compound is electrophilic and thus sensitive to nucleophiles, including bases: : Phosgene oxime has been used to prepare heterocycles that contain N-O bonds, such as isoxaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Oxides
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide *Nitrogen dioxide (), nitrogen(IV) oxide *Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide *Dinitrogen dioxide (), nitrogen(II) oxide Dimer (chemistry), dimer *Dinitrogen trioxide (), nitrogen(II,IV) oxide *Dinitrogen tetroxide (), nitrogen(IV) oxide Dimer (chemistry), dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate *Nitrosyl azide (), nitrogen(−I,0,I,II) oxide *Nitryl azide () *Oxatetrazole () *Trinitramide ( or ), nitrogen(0,IV) oxide Anions *Nitroxyl, Nitroxide () *Nitrite ( or ) *Nitrate () *Peroxynitrite ( or ) *Peroxynitrate ( or ) *Orthonitrate (, analogous to phosphate ) *Hyponitrite ( or ) *Trioxodinitrate or hyponitrate ( or ) *Nitroxylate ( or ) *Ammonium dinitramide, Dinitramide ( or ) Cations *Nitrosonium ( or ) *Nitronium ( or ) Atm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosyl Chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound. Structure and synthesis The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°. Production Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine and nitric o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |