|
Trefoil Knot Fold
The trefoil knot fold is a protein fold in which the protein backbone is twisted into a trefoil knot shape. "Shallow" knots in which the tail of the polypeptide chain only passes through a loop by a few residues are uncommon, but "deep" knots in which many residues are passed through the loop are extremely rare. Deep trefoil knots have been found in the SPOUT superfamily.Zarembinski TI, Kim Y, Peterson K, Christendat D, Dharamsi A, Arrowsmith CH, Edwards AM, Joachimiak A. (2003). Deep trefoil knot implicated in RNA binding found in an archaebacterial protein. ''Proteins'' 50(2):177-83 including methyltransferase proteins involved in posttranscriptional RNA modification in all three domains of life, including bacterium ''Thermus thermophilus''Nureki O, Shirouzu M, Hashimoto K, Ishitani R, Terada T, Tamakoshi M, Oshima T, Chijimatsu M, Takio K, Vassylyev DG, Shibata T, Inoue Y, Kuramitsu S, Yokoyama S. (2002). An enzyme with a deep trefoil knot for the active-site architecture. ''A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TFF3
Trefoil factor 3 is a protein that in humans is encoded by the ''TFF3'' gene. Function Members of the trefoil family are characterized by having at least one copy of the trefoil motif, a 40-amino acid domain that contains three conserved disulfide bonds. They are stable secretory proteins expressed in gastrointestinal mucosa. Their functions are diverse, including protection of the mucosa, thickening of the mucus, and increasing epithelial healing rates. This gene is a marker of columnar epithelium and is expressed in a variety of tissues including goblet cells of the intestines and colon. This gene and two other related trefoil family member genes are found in a cluster on chromosome 21. Glycan binding All three human trefoil factors are lectins that interact specifically with the disaccharide GlcNAc-α-1,4-Gal. This disaccharide is an unusual glycotope that is only known to exist on the large, heavily glycosylated, mucins in the mucosa. By cross-linking mucins Mucins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
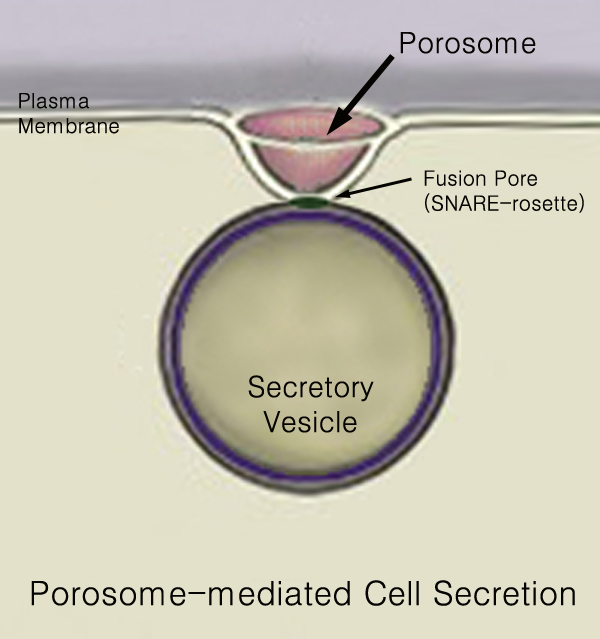
Secretion
440px Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. Secretion in bacterial species means the transport or translocation of effector molecules for example: proteins, enzymes or toxins (such as cholera toxin in pathogenic bacteria e.g. ''Vibrio cholerae'') from across the interior (cytoplasm or cytosol) of a bacterial cell to its exterior. Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment for adaptation and survival. In eukaryotic cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastric Acid
Gastric acid, gastric juice, or stomach acid is a digestive fluid formed within the stomach lining. With a pH between 1 and 3, gastric acid plays a key role in digestion of proteins by activating digestive enzymes, which together break down the long chains of amino acids of proteins. Gastric acid is regulated in feedback systems to increase production when needed, such as after a meal. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid, ensuring a regulated pH. These cells also produce mucus – a viscous barrier to prevent gastric acid from damaging the stomach. The pancreas further produces large amounts of bicarbonate and secretes bicarbonate through the pancreatic duct to the duodenum to neutralize gastric acid passing into the digestive tract. The active components of gastric acid are protons and chloride. Often simplistically described as hydrochloric acid, these species are produced by parietal cells in the gastric glands in the stomach. The sec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motility
Motility is the ability of an organism to move independently, using metabolic energy. Definitions Motility, the ability of an organism to move independently, using metabolic energy, can be contrasted with sessility, the state of organisms that do not possess a means of self-locomotion and are normally immobile. Motility differs from mobility, the ability of an object to be moved. The term vagility encompasses both motility and mobility; sessile organisms including plants and fungi often have vagile parts such as fruits, seeds, or spores which may be dispersed by other agents such as wind, water, or other organisms. Motility is genetically determined, but may be affected by environmental factors such as toxins. The nervous system and musculoskeletal system provide the majority of mammalian motility. In addition to animal locomotion, most animals are motile, though some are vagile, described as having passive locomotion. Many bacteria and other microorganisms, and multicellu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TFF2
Trefoil factor 2 is a protein that in humans is encoded by the ''TFF2'' gene. Members of the trefoil family are characterized by having at least one copy of the trefoil motif, a 40-amino acid domain that contains three conserved disulfides. They are stable secretory proteins expressed in gastrointestinal mucosa. Their functions are not defined, but they may protect the mucosa from insults, stabilize the mucus layer and affect healing of the epithelium. The encoded protein inhibits gastric acid secretion. This gene and two other related trefoil family member genes are found in a cluster on chromosome 21. Glycan binding All human trefoil factors are lectins that interact specifically with the disaccharide GlcNAc-α-1,4-Gal. This disaccharide is an unusual glycotope that is only known to exist on the large, heavily glycosylated, mucins in the mucosa. By cross-linking mucins Mucins () are a family of high molecular weight, heavily glycosylated proteins (glycoconjugates) produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stomach Mucosa
The gastric mucosa is the mucous membrane layer of the stomach, which contains the glands and the gastric pits. In humans, it is about 1 mm thick, and its surface is smooth, soft, and velvety. It consists of simple columnar epithelium, lamina propria, and the muscularis mucosae. Description In its fresh state, it is of a pinkish tinge at the pyloric end and of a red or reddish-brown color over the rest of its surface. In infancy it is of a brighter hue, the vascular redness being more marked. It is thin at the cardiac extremity, but thicker toward the pylorus. During the contracted state of the organ it is thrown into numerous plaits or rugae, which, for the most part, have a longitudinal direction, and are most marked toward the pyloric end of the stomach, and along the greater curvature. These folds are entirely obliterated when the organ becomes distended. When examined with a lens, the inner surface of the mucous membrane presents a peculiar honeycomb appearance from b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure Prediction
Protein structure prediction is the inference of the three-dimensional structure of a protein from its amino acid sequence—that is, the prediction of its secondary and tertiary structure from primary structure. Structure prediction is different from the inverse problem of protein design. Protein structure prediction is one of the most important goals pursued by computational biology; and it is important in medicine (for example, in drug design) and biotechnology (for example, in the design of novel enzymes). Starting in 1994, the performance of current methods is assessed biannually in the CASP experiment (Critical Assessment of Techniques for Protein Structure Prediction). A continuous evaluation of protein structure prediction web servers is performed by the community project CAMEO3D. Protein structure and terminology Proteins are chains of amino acids joined together by peptide bonds. Many conformations of this chain are possible due to the rotation of the main chain abou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |