|
Transition Metal Nitrite Complex
In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite () ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle. Structure and bonding Bonding modes Three linkage isomers are common for nitrite ligands, O-bonded, N-bonded, and bidentate O,O-bonded. The former two isomers have been characterized for the pentamminecobalt(III) system, i.e. and , referred to as N-nitrito and O-nitrito, respectively. These two forms are sometimes called nitro and nitrito. An example of chelating nitrite is – "bipy" is the bidentate ligand 2,2′-bipyridyl. This bonding mode is sometimes described as κ2O,O-. Focusing on electron-counting in monometallic complexes, O-bonded, N-bonded are viewed as 1-electron pseudohalides ("X-ligand"). The bidentate O,O-bonded is an "L-X ligand", akin to bidentate car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
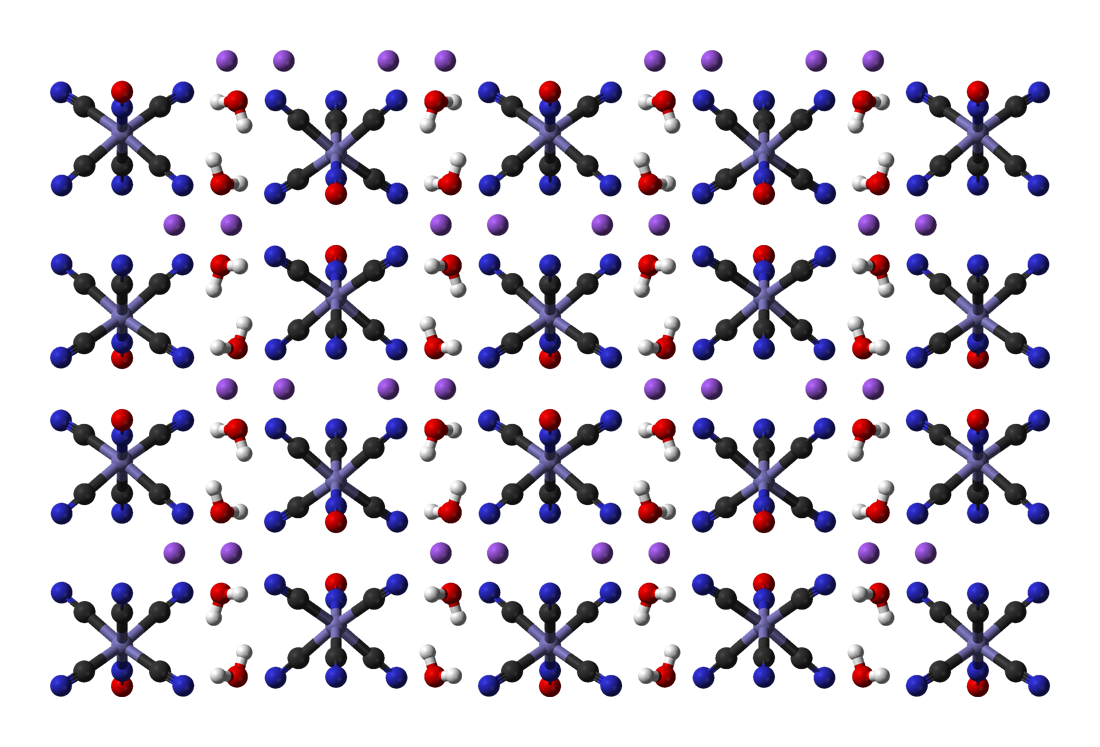
Sodium Cobaltinitrite
Sodium hexanitritocobaltate(III) is inorganic compound with the formula . The anion of this yellow-coloured salt consists of the transition metal nitrite complex . It was a reagent for the qualitative test for potassium and ammonium ions. Synthesis and reactions The compound is prepared by oxidation of cobalt(II) salts in the presence of sodium nitrite Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite ...: : Application for analysis of potassium Although the sodium cobaltinitrite is soluble in water, it forms the basis of a quantitative determination of potassium, thallium, and ammonium ions. Under the recommended reaction conditions the insoluble double salt, is precipitated and weighed. In geochemical analysis, sodium cobaltinitrite is used to distinguish alkali feldspars from p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isonitrosyl
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat complexes that contain nitric oxide">hypertension. Metal nitrosyl complexes are complex (chemistry)">complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand. Bonding and structure Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxide, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This trend is illustrated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Nitrosyl Complex
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat hypertension. Metal nitrosyl complexes are complex (chemistry), complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand. Bonding and structure Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxide, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This trend is illustrated by the isoelectronic pair Fe(CO)2(NO)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite Reductase
Nitrite reductase refers to any of several classes of enzymes that catalyze the reduction of nitrite. There are two classes of NIR's. A multi haem enzyme reduces NO2− to a variety of products. Copper containing enzymes carry out a single electron transfer to produce nitric oxide. Iron based There are several types of iron based enzymes. Cytochrome cd1, or ''Pseudomonas'' cytochrome oxidase contains two c and two d type hemes with two polypeptide chains. Different forms of this reductase catalyze the formation of nitric oxide or nitrous oxide. A version of this compound was originally called oxidoreductase.html"_;"title="errocytochrome_c-551:oxidoreductase">errocytochrome_c-551:oxidoreductase_It_was_initially_considered_an_oxidase.__It_catalyzes_the_reduction_of_NO2−_to_NO.__This_tetraheme_enzyme_has_two_Protein_subunit.html" ;"title="oxidoreductase.html" ;"title="oxidoreductase.html" ;"title="errocytochrome c-551:oxidoreductase">errocytochrome c-551:oxidoreductase">oxido ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver. In biochemical terms, heme is a coordination complex "consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands." The definition is loose, and many depictions omit the axial ligands. Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase. The word ''haem'' is derived from Greek ''haima'' meaning "blood". Function Hemoproteins have diverse biological functions incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite Oxidoreductase
Nitrite oxidoreductase (NOR or NXR) is an enzyme involved in nitrification. It is the last step in the process of aerobic ammonia oxidation, which is carried out by two groups of nitrifying bacteria: ammonia oxidizers such as '' Nitrosospira'', ''Nitrosomonas'' and '' Nitrosococcus'' convert ammonia to nitrite, while nitrite oxidizers such as ''Nitrobacter'' and ''Nitrospira'' oxidize nitrite to nitrate. The enzyme is bound to the inner cytoplasmic surface of the bacterial membrane and contains multiple subunits, iron-sulfur centers and a molybdenum cofactor. The enzyme is relatively abundant, making up 10-30% of the total protein in these bacteria and forms densely packed structures on the membrane surface. ;Reaction : + acceptor + reduced\ acceptor See also *Microbial metabolism Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ... with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Structure The ion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosyl Complex
Sodium nitroprusside, a medicinally significant metal nitrosyl-pentacyanoferrate (Fe-III) compound, used to treat complexes that contain nitric oxide">hypertension. Metal nitrosyl complexes are complex (chemistry)">complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand. Bonding and structure Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxide, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This trend is illustrated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroprusside
Sodium nitroprusside (SNP), sold under the brand name Nitropress among others, is a medication used to lower blood pressure. This may be done if the blood pressure is very high and resulting in symptoms, in certain types of heart failure, and during surgery to decrease bleeding. It is used by continuous injection into a vein. Onset is nearly immediate and effects last for up to ten minutes. It is available as a generic medication. Side effects and mechanism Common side effects include low blood pressure and cyanide toxicity. Other serious side effects include methemoglobinemia. It is not generally recommended during pregnancy due to concerns of side effects. High doses are not recommended for more than ten minutes. It works by increasing nitric oxide levels in the blood, which increases cGMP levels in cells, and causes dilation of blood vessels. History, society and culture Sodium nitroprusside was discovered as early as 1850 and found to be useful in medicine in 1928. It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)

(ON)(pyridine)4 _(9RUKQOE02).png)


-3D-balls.png)