|
Transient Receptor Potential Calcium Channel Family
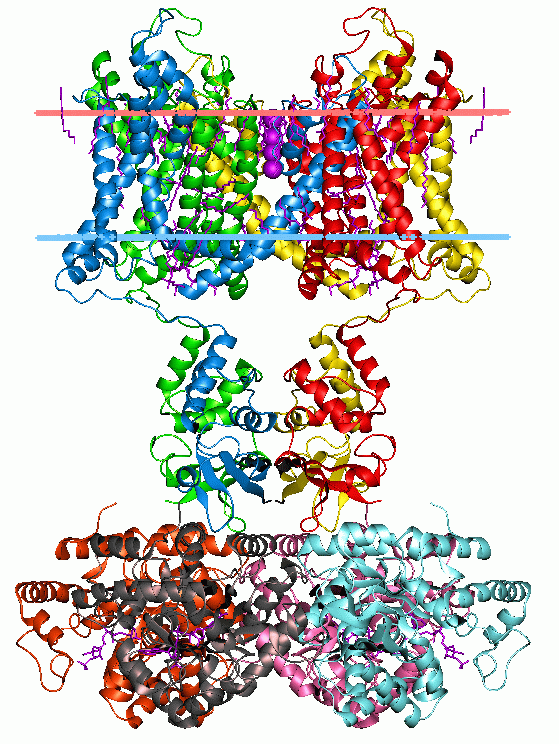
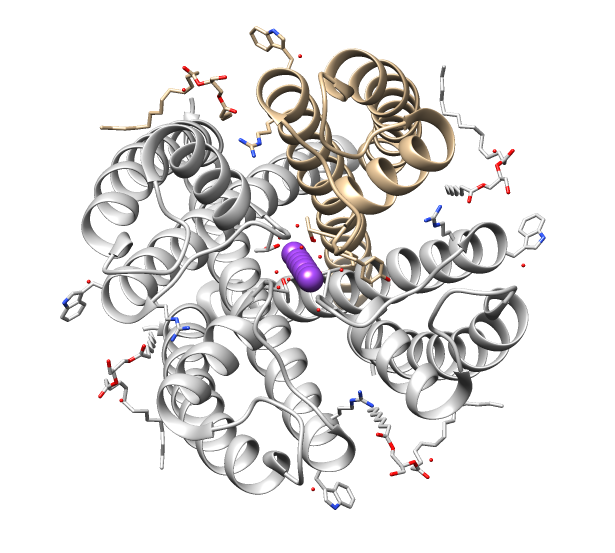
The transient receptor potential Ca2+ channel (TRP-CC) familyTC# 1.A.4 is a member of the Voltage-gated ion channel, voltage-gated ion channel (VIC) superfamily and consists of cation channels conserved from worms to humans. The TRP-CC family also consists of seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML) based on their amino acid sequence homology: # the canonical or classic TRPs, # the vanilloid receptor TRPs, # the melastatin or long TRPs, # ankyrin (whose only member is the transmembrane protein 1 [TRPA1]) # TRPN after the nonmechanoreceptor potential C (nonpC), and the more distant cousins, # the polycystins # and mucolipins. A representative list of members belonging to the TRP-CC family can be found in thTransporter Classification Database Function Members of the TRP-CC family are characterized as cellular sensors with polymodal activation and gating properties. Many TRP channels are activated by a variety of different stimuli and function as signal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage-gated Ion Channel
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nociception
Nociception (also nocioception, from Latin ''nocere'' 'to harm or hurt') is the sensory nervous system's process of encoding noxious stimuli. It deals with a series of events and processes required for an organism to receive a painful stimulus, convert it to a molecular signal, and recognize and characterize the signal in order to trigger an appropriate defense response. In nociception, intense chemical (e.g., capsaicin present in Chili pepper or Cayenne pepper), mechanical (e.g., cutting, crushing), or thermal (heat and cold) stimulation of sensory neurons called nociceptors produces a signal that travels along a chain of nerve fibers via the spinal cord to the brain. Nociception triggers a variety of physiological and behavioral responses to protect the organism against an aggression and usually results in a subjective experience, or perception, of pain in sentient beings. Detection of noxious stimuli Potentially damaging mechanical, thermal, and chemical stimuli are detected ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Transporters
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Proteins
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Proteins
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the surv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein familie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Channelopathy
Channelopathies are a group of diseases caused by the dysfunction of ion channel subunits or their interacting proteins. These diseases can be inherited or acquired by other disorders, drugs, or toxins. Mutations in genes encoding ion channels, which impair channel function, are the most common cause of channelopathies. There are more than 400 genes that encode ion channels, found in all human cell types and are involved in almost all physiological processes. Each type of channel is a multimeric complex of subunits encoded by a number of genes. Depending where the mutation occurs it may affect the gating, conductance, ion selectivity, or signal transduction of the channel. Channelopathies can be categorized based on the organ system which they are associated with. In the cardiovascular system, the electrical impulse needed for each heartbeat is made possible by the electrochemical gradient of each heart cell. Because the heartbeat is dependent on the proper movement of ions across ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autophosphorylation
Autophosphorylation is a type of post-translational modification of proteins. It is generally defined as the phosphorylation of the kinase by itself. In eukaryotes, this process occurs by the addition of a phosphate group to serine, threonine or tyrosine residues within protein kinases, normally to regulate the catalytic activity.Petsko, GA and Ringe, D 2009, 'Protein Structure and Function', Oxford University Press Inc., New York, U.S.A Autophosphorylation may occur when a kinases' own active site catalyzes the phosphorylation reaction (cis autophosphorylation), or when another kinase of the same type provides the active site that carries out the chemistry (trans autophosphorylation). The latter often occurs when kinase molecules dimerize. In general, the phosphate groups introduced are gamma phosphates from nucleoside triphosphates, most commonly ATP. Function Protein kinases, many of which are regulated by autophosphorylation, are vital in controlling the cellular prolifer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ankyrin Repeat
The ankyrin repeat is a 33-residue motif in proteins consisting of two alpha helices separated by loops, first discovered in signaling proteins in yeast Cdc10 and ''Drosophila'' Notch. Domains consisting of ankyrin tandem repeats mediate protein–protein interactions and are among the most common structural motifs in known proteins. They appear in bacterial, archaeal, and eukaryotic proteins, but are far more common in eukaryotes. Ankyrin repeat proteins, though absent in most viruses, are common among poxviruses. Most proteins that contain the motif have four to six repeats, although its namesake ankyrin contains 24, and the largest known number of repeats is 34, predicted in a protein expressed by ''Giardia lamblia''. Ankyrin repeats typically fold together to form a single, linear solenoid structure called ankyrin repeat domains. These domains are one of the most common protein–protein interaction platforms in nature. They occur in a large number of functionally diverse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanilloid Receptor
TRPV is a family of transient receptor potential cation channels (TRP channels) in animals. All TRPVs are highly calcium selective. TRP channels are a large group of ion channels consisting of six protein families, located mostly on the plasma membrane of numerous human and animal cell types, and in some fungi. TRP channels were initially discovered in the ''trp'' mutant strain of the fruit fly ''Drosophila'' that displayed transient elevation of potential in response to light stimuli, and were therefore named "transient receptor potential" channels. The name now refers only to a family of proteins with similar structure and function, not to the mechanism of their activation. Later, TRP channels were found in vertebrates where they are ubiquitously expressed in many cell types and tissues. There are about 28 TRP channels that share some structural similarity to each other. These are grouped into two broad groups: group 1 includes TRPC ( "C" for canonical), TRPV ("V" for vanillo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |