|
Thiete
Thiete is a heterocyclic compound containing an unsaturated four-membered ring with three carbon atoms and one sulfur atom. It is more commonly encountered not on its own, but in anellated derivatives, several of which have been synthesized. Thietes are generally not very stable. Structure Thiete is a valence isomer of the compound thioacrolein (CH2=CHCH=S) and undergoes ring opening to it at temperatures below 400°C. Thiete has been shown to be planar, with a C-S-C angle of 76.8 degrees. Derivatives Benzothietes are thietes annulated to benzo group. Such species are prepared by flash vacuum pyrolysis of 2-mercaptobenzyl alcohols. They are precursors to other S-heterocycles. Thiete 1,1-dioxides are sulfones, the parent being C3H4SO2. They are more stable than the parent thietes.Thomas C. Sedergran and Donald C. Dittmer "Thiete 1,1-dioxide and Chlorothiete 1,1-dioxide" Org. Synth. 1984, vol. 62, 210. Substituted thiete-1,1-dioxides can also be prepared by +2cycloaddition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiete
Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated. Unsubstituted 1,2-dithiete has been generated in thermolytic reactions and was characterized by microwave spectroscopy, ultraviolet photoelectron spectroscopy and infrared spectroscopy in a low temperature matrix. The open ring isomer, dithioglyoxal, HC(S)C(S)H, is less stable than the 1,2-dithiete. The dithione can be prepared (as ''trans''-dithioglyoxal) by low temperature photolysis of 1,3-dithiol-2-one. Quantum chemical calculations reproduce the observed greater stability of 1,2-dithiete only if large basis-sets with polarization functions are used. : See also * Dithietane - the corresponding saturated ring * Thiete Thiete is a heterocyclic compound contai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfene
Sulfene is an extremely reactive chemical compound with the formula H2C=SO2. It is the simplest member of the sulfenes, the group of compounds which are ''S'',''S''-dioxides of thioaldehydes and thioketones, and have the general formula R2C=SO2. Preparation The first general method for preparation of sulfene as an intermediate, reported simultaneously in 1962 by Gilbert Stork and by Günther Optiz, involved removal of hydrogen chloride from methanesulfonyl chloride using triethylamine in the presence of an enamine as trapping agent. The formation of a thietane 1,1-dioxide derivative was taken as evidence for the intermediacy of sulfene. Because of the highly electrophilic character of sulfene, the use of amines presents difficulties, since they can intercept the sulfene to form adducts. A simple alternative which avoids the use of amines involves desilylation of trimethylsilylmethanesulfonyl chloride with cesium fluoride in the presence of trapping agents. : (CH3)3SiCH2SO2Cl + Cs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated And Unsaturated Compounds
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis acids and bases, Lewis base. The term is used in many contexts and for many classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'. Organic chemistry Unsaturated compounds generally carry out typical addition reactions that are not possible with saturated compounds such as alkanes. A saturated organic compound has only single bonds between carbon atoms. An important class of saturated compounds are the alkanes. Many saturated compounds have functional groups, e.g., alcohols. Unsaturated organic compounds The concept of saturation can be described using various naming systems, formulas, and Analytical chemistry, analytical tests. For instance, IUPAC nomenclature of organic chemistry, IU ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Compound
In the field of organic chemistry, a polycyclic compound is an organic compound featuring several closed rings of atoms, primarily carbon. These ring substructures include cycloalkanes, aromatics, and other ring types. They come in sizes of three atoms and upward, and in combinations of linkages that include tethering (such as in biaryls), fusing (edge-to-edge, such as in anthracene and steroids), links via a single atom (such as in spiro compounds), bridged compounds, and longifolene. Though poly- literally means "many", there is some latitude in determining how many rings are required to be considered polycyclic; many smaller rings are described by specific prefixes (e.g., bicyclic, tricyclic, tetracyclic, etc.), and so while it can refer to these, the title term is used with most specificity when these alternative names and prefixes are unavailable. In general, the term polycyclic includes polycyclic aromatic compounds, including polycyclic aromatic hydrocarbons, as well as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
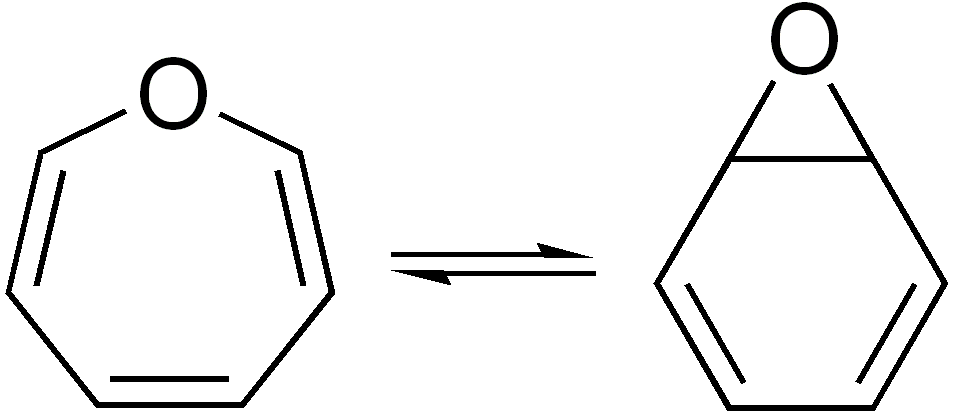
Valence Isomer
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions. Benzene There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally proposed for benzene itself before the actual structure of benzene was known. Others were later synthesized in lab. Some have been observed to isomerize to benzene, whereas others tend to undergo other reactions instead, or isomerize by ways other than pericyclic reactions. Image:Benzene-2D-flat.png, Benzene Image:Historic Benzene Formulae Dewar(1867) V.1.svg, Dewar benzene Image:Prisman2.svg, Prismane Image:Benzvalene.png, Benzvalene Image:Bicycloprop-2-enyl.svg, Bicyclopropenyl Cyclooctatetraene The valence isomers are not restricted to isomers of benzene. Valence isomers are also seen in the series (CH)8. Due to the larger number of units, the number of possible valence isomers is also greater and at least 21: Image:Cyclooctatet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrolein
Acrolein (systematic name: propenal) is the simplest unsaturated aldehyde. It is a colorless liquid with a piercing, acrid smell. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fat breaking down into acrolein. It is produced industrially from propylene and mainly used as a biocide and a building block to other chemical compounds, such as the amino acid methionine. History Acrolein was first named and characterized as an aldehyde by the Swedish chemist Jöns Jacob Berzelius in 1839. He had been working with it as a thermal degradation product of glycerol, a material used in the manufacture of soap. The name is a contraction of ‘acrid’ (referring to its pungent smell) and ‘oleum’ (referring to its oil-like consistency). In the 20th century, acrolein became an important intermediate for the industrial production of acrylic acid and acrylic plastics. Production Acrolein is prepared industrially by oxidation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flash Vacuum Pyrolysis
Flash vacuum pyrolysis (FVP) is a technique in organic synthesis. It entails heating a precursor molecule intensely and briefly. Two key parameters are the temperature and duration (or residence time), which are adjusted to optimize yield, conversion, and avoidance of intractable products. Often the experiment entails volatilizing a precursor, which is drawn through a "hot zone" followed by rapid condensation. The apparatus typically is conducted under dynamic vacuum. The hot zone must impart heat to the gaseous molecules, so it is generally packed with solids to induce gas-solid collisions. The packing material is generally chemically inert, such as quartz. The precursor (i) volatilizes with gentle heating and under vacuum, (ii) the precursor fragments or rearranges in the hot zone, and finally (iii) the products are collected by rapid cooling. Rapid post-reaction cooling and the dilution inherent in gases both suppress bimolecular degradation pathways. Examples The technique ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Alcohol
Benzyl alcohol is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide. Natural occurrences Benzyl alcohol is produced naturally by many plants and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth and ylang-ylang. It is also found in castoreum from the castor sacs of beavers. Benzyl esters also occur naturally. Preparation Benzyl alcohol is produced industrially from toluene via benzyl chloride, which is hydrolyzed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents. Synthesis and reactions By oxidation of thioethers and sulfoxides Sulfones are typically prepared by organic oxidation of thioethers, often referred to as sulfides. Sulfoxides are intermediates in this route. For example, dimethyl sulfide oxidizes to dimethyl sulfoxide and then to dimethyl sulfone. From SO2 : Sulfur dioxide is a convenient and widely used source of the sulfonyl functional group. Specifically, Sulfur dioxide participates in cycloaddition reactions with dienes. The industrially useful solvent sulfolane is prepared by addition of sulfur dioxide to buta-1,3-diene followed by hydrogenation of the resulting sulfolene. From sulfonyl and sulfuryl halides Sulfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Heterocycles
Sulfur (or sulphur in British English) is a chemical element with the Symbol (chemistry), symbol S and atomic number 16. It is abundance of the chemical elements, abundant, Polyvalency (chemistry), multivalent and nonmetallic. Under standard conditions for temperature and pressure, normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula octasulfur, S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native element minerals, native form, sulfur on Earth usually occurs as sulfide minerals, sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, history of China#Ancient China, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". To ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |