|
Thick Ascending Limb Of The Loop Of Henle
Within the nephron of the kidney, the ascending limb of the loop of Henle is a segment of the heterogenous loop of Henle downstream of the descending limb, after the sharp bend of the loop. This part of the renal tubule is divided into a thin and thick ascending limb; the thick portion is also known as the distal straight tubule, in contrast with the distal convoluted tubule downstream. Structure The ascending limb of the loop of Henle is a direct continuation from the descending limb of loop of Henle, and one of the structures in the nephron of the kidney. The ascending limb has a thin and a thick segment. The ascending limb drains urine into the distal convoluted tubule. The thin ascending limb is found in the medulla of the kidney, and the thick ascending limb can be divided into a part that is in the renal medulla and a part that is in the renal cortex. The ascending limb is much thicker than the descending limb. At the junction of the thick ascending limb and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nephron
The nephron is the minute or microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure called Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 1 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the foot processes of the podocytes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged (some are added, others are removed); first with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
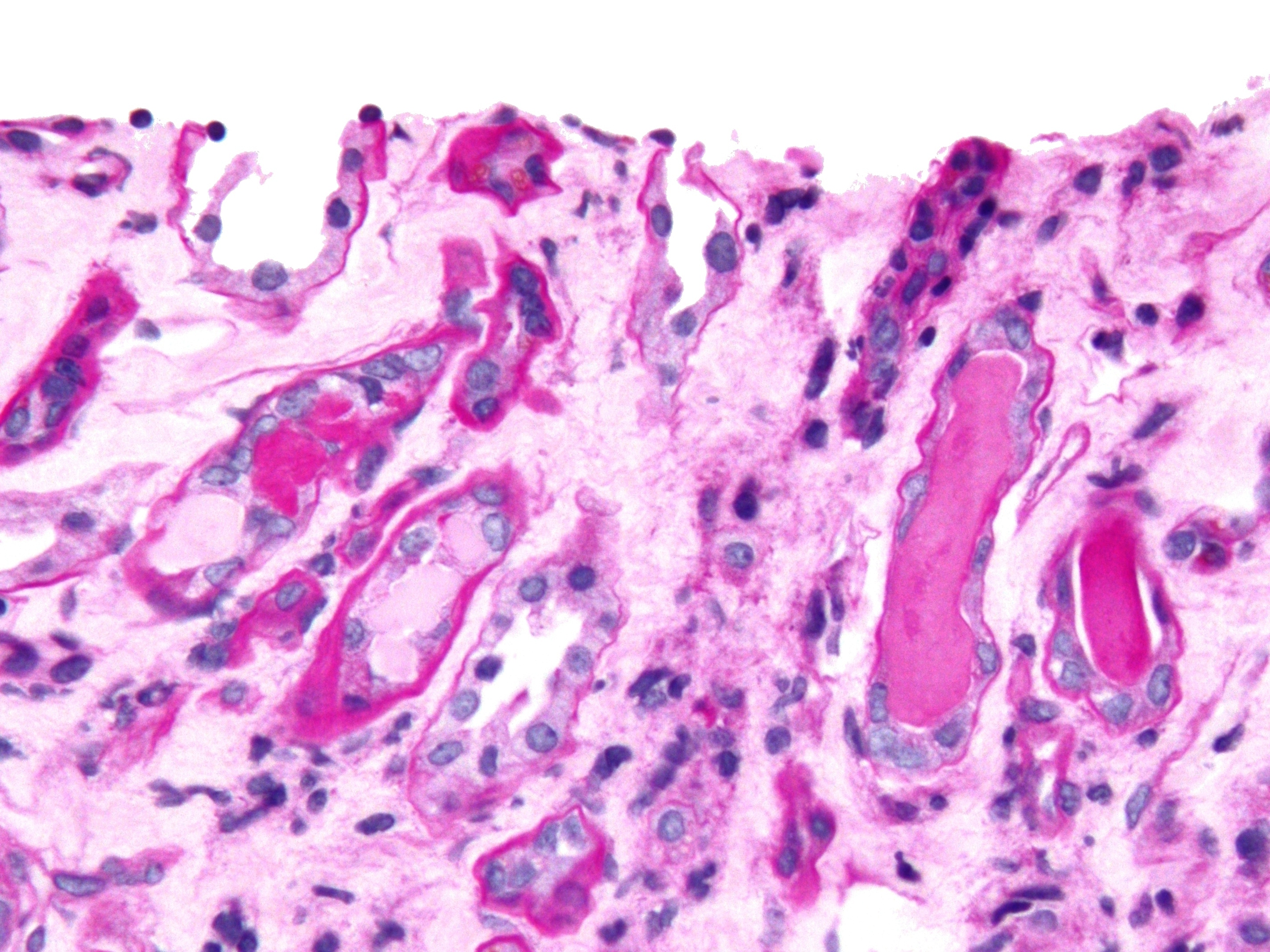
Urinary Casts
Urinary casts are microscopic cylindrical structures produced by the kidney and present in the urine in certain disease states. They form in the distal convoluted tubule and collecting ducts of nephrons, then dislodge and pass into the urine, where they can be detected by microscopy. They form via precipitation of Tamm–Horsfall mucoprotein, which is secreted by renal tubule cells, and sometimes also by albumin in conditions of proteinuria. Cast formation is pronounced in environments favoring protein denaturation and precipitation (low flow, concentrated salts, low pH). Tamm–Horsfall protein is particularly susceptible to precipitation in these conditions. Casts were first described by Henry Bence Jones (1813–1873). As reflected in their cylindrical form, casts are generated in the small distal convoluted tubules and collecting ducts of the kidney, and generally maintain their shape and composition as they pass through the urinary system. Although the most common forms a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tamm-Horsfall Protein
Uromodulin (UMOD), also known as Tamm–Horsfall protein (THP), is a Zona pellucida-like domain-containing glycoprotein that in humans is encoded by the ''UMOD'' gene. Uromodulin is the most abundant protein excreted in ordinary urine. Gene The human UMOD gene is located on chromosome 16. While several transcript variants may exist for this gene, the full-length natures of only two have been described to date. These two represent the major variants of this gene and encode the same isoform. Protein THP is a GPI-anchored glycoprotein. It is not derived from blood plasma but is produced by the thick ascending limb of the loop of Henle of the mammalian kidney. While the monomeric molecule has a MW of approximately 85 kDa, it is physiologically present in urine in large aggregates of up to several million Da. When this protein is concentrated at low pH, it forms a gel. Uromodulin represents the most abundant protein in normal human urine (results based on MSMS determinations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Potential
In electrochemistry, the electrochemical potential (ECP), ', is a thermodynamic measure of chemical potential that does not omit the energy contribution of electrostatics. Electrochemical potential is expressed in the unit of J/ mol. Introduction Each chemical species (for example, "water molecules", "sodium ions", "electrons", etc.) has an electrochemical potential (a quantity with units of energy) at any given point in space, which represents how easy or difficult it is to add more of that species to that location. If possible, a species will move from areas with higher electrochemical potential to areas with lower electrochemical potential; in equilibrium, the electrochemical potential will be constant everywhere for each species (it may have a different value for different species). For example, if a glass of water has sodium ions (Na+) dissolved uniformly in it, and an electric field is applied across the water, then the sodium ions will tend to get pulled by the electric fie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tonicity
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution. Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''.A hypotonic s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium–hydrogen Antiporter 3
Sodium–hydrogen antiporter 3 also known as sodium–hydrogen exchanger 3 (NHE3) or solute carrier family 9 member 3 (SLC9A3) is a protein that in humans is encoded by the ''SLC9A3'' gene. SLC9A3 is a sodium–hydrogen antiporter. It is found on the apical side of the epithelial cells of the proximal tubule of the nephron of the kidney, in the apical membrane of enterocytes of the intestine, as well as the basolateral side of both duodenal and pancreatic cells responsible for the release of HCO−3 into the duodenal lumen. It is primarily responsible for maintaining the balance of sodium. It is also indirectly linked to buffering of blood pH. The NHE3 antiporter imports one sodium ion into the cytosol of a tubule cell as it ejects one hydrogen ion from the cell into the lumen of the proximal tubule. The sodium within the tubule cell may then be retained by the body rather than excreted in the urine. The NHE3 antiporter indirectly contributes to blood buffering capacity bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Transport
In cellular biology, ''active transport'' is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate ( ATP), and secondary active transport that uses an electrochemical gradient. Some examples of active transport include: * Phagocytosis of bacteria by macrophages * Movement of calcium ions out of cardiac muscle cells * Transportation of amino acids across the intestinal lining in the human gut * Secretion of proteins such as enzymes, peptide hormones, and antibodies from various cells * Functioning of white blood cells to defend invading diseases Active cellular transportation (ACT) Unlike passive transport, which uses the kinetic energy and natural entropy of molecules moving down a gradient, active ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |