|
Thermospermine
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature. Natural polyamines Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC). Polyamines are found in high concentrations in the mammalian brain. File:Spermidine-2D-skeletal.svg, spermidine File:Spermine.svg, spermine Synthetic polyamines Several synthetic polyamines are used in chemical industry and the research laboratory. They are mainly of interest as additives to motor oil and as co-r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Chemistry
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazinane
Triazinanes are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is . They exist in three isomeric forms, 1,3,5-triazinanes being common. The triazinanes have six-membered cyclohexane-like ring but with three carbons replaced by nitrogens. Most commonly, the amines are tertiary. References * ''Heterocyclic Chemistry'' T.L. Gilchrist 1985 (1997, ) See also * 6-membered rings with one nitrogen atom: Piperidine * 6-membered rings with two nitrogen atoms: Diazinane ** Hexahydropyrimidine ** Hexahydropyridazine * Triazine Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is . They exist in three isomeric forms, 1,3,5-triazines being common. Structure The triazines have planar six-membered benzene-like ring but ... {{Authority control Heterocyclic compounds with 1 ring Nitrogen heterocycles Six-membered rings Polyamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylenimine
Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon aliphatic ''CHCH'' spacers. Linear polyethyleneimines contain all secondary amines, in contrast to branched PEIs which contain primary, secondary and tertiary amino groups. Totally branched, dendrimeric forms were also reported. PEI is produced on an industrial scale and finds many applications usually derived from its polycationic character. Properties The linear PEI is a semi-crystalline solid at room temperature while branched PEI is a fully amorphous polymer existing as a liquid at all molecular weights. Linear polyethyleneimine is soluble in hot water, at low pH, in methanol, ethanol, or chloroform. It is insoluble in cold water, benzene, ethyl ether, and acetone. Linear polyethyleneimine has a melting point of around 67 °C. Both linear and branched polyethyleneimine can be stored at room temperature. Linear polyethyleneimine is able to form cryogels up ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
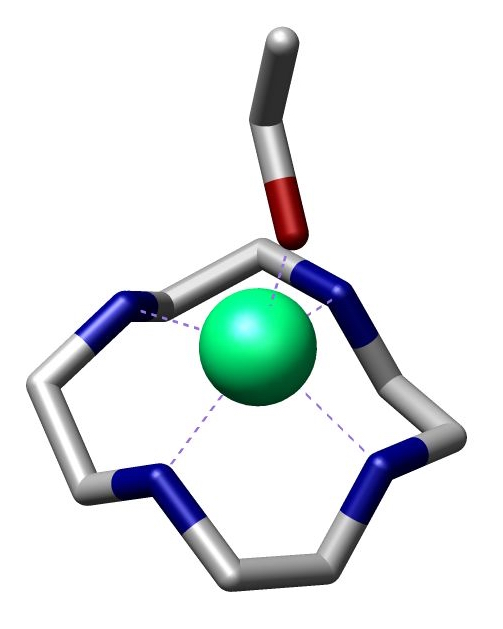
Cyclen
Cyclen (1,4,7,10-tetraazacyclododecane) is a aza-crown ether with the formula (CH2CH2NH)4. It is a white solid. Synthesis Some syntheses exploit the Thorpe-Ingold effect to facilitate ring-formation. Illustrative is the reaction of the deprotonated tosylamides with ditosylates: :TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4 The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine. High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL. : In one study cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivatives, also referred to as aziridines, are of broader interest in medicinal chemistry. Structure The bond angles in aziridine are approximately 60°, considerably less than the normal hydrocarbon bond angle of 109.5°, which results in angle strain as in the comparable cyclopropane and ethylene oxide molecules. A banana bond model explains bonding in such compounds. Aziridine is less basic than acyclic aliphatic amines, with a pKa of 7.9 for the conjugate acid, due to increased s character of the nitrogen free electron pair. Angle strain in aziridine also increases the barrier to nitrogen inversion. This barrier height permits the isolation of separate ''invertomers'', for example the ''cis'' and ''trans'' invertomers of ''N''-chloro- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylenimine
Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon aliphatic ''CHCH'' spacers. Linear polyethyleneimines contain all secondary amines, in contrast to branched PEIs which contain primary, secondary and tertiary amino groups. Totally branched, dendrimeric forms were also reported. PEI is produced on an industrial scale and finds many applications usually derived from its polycationic character. Properties The linear PEI is a semi-crystalline solid at room temperature while branched PEI is a fully amorphous polymer existing as a liquid at all molecular weights. Linear polyethyleneimine is soluble in hot water, at low pH, in methanol, ethanol, or chloroform. It is insoluble in cold water, benzene, ethyl ether, and acetone. Linear polyethyleneimine has a melting point of around 67 °C. Both linear and branched polyethyleneimine can be stored at room temperature. Linear polyethyleneimine is able to form cryogels up ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-Tris(aminomethyl)ethane
1,1,1-Tris(aminomethyl)ethane (TAME) is an organic compound with the formula CHC(CHNH). It is a colorless liquid. It is classified as a polyamine tripodal ligand, i.e., capable of binding to metal ions through three sites and hence is a tridentate chelating ligand, occupying a face of the coordination polyhedron. Preparation TAME is synthesized by the Pd/C-catalyzed hydrogenation of 1,1,1-tris(azidomethyl)ethane. Although azides are potentially explosive, they are excellent and practical source of primary amines. The required tris(azidomethyl)ethane is obtained from the tritosylate by salt metathesis using sodium azide. These two steps are: :3 NaN + CHC(CHOTs) → CHC(CHN) + 3 NaOTs :3 H + CHC(CHN) → CHC(CHNH) + 3 N Complexes of TAME The tripodal TAME ligand coordinates facially to metal ions. This stereochemical feature has been exploited in the preparation of platinum(IV) cage complexes, e.g., t(tame) which is a six coordinate Pt(IV) complex. Platinum in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(2-aminoethyl)amine
Tris(2-aminoethyl)amine is the organic compound with the chemical formula, formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Abbreviated tren or TREN it is a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry. Tren is a C3-symmetric, tetradentate chelating ligand that forms stable complexes with transition metals, especially those in the 2+ and 3+ oxidation states. Tren complexes exist with relatively few isomers, reflecting the constrained connectivity of this tetramine. Thus, only a single achiral stereoisomer exists for [Co(tren)X2]+, where X is halide or pseudohalide. In contrast, for [Co(trien)X2]+ five diastereomers are possible, four of which are chiral. In a few cases, tren serves as a tridentate ligand with one of the primary amine groups non-coordinated. Tren is a common impurity in the more common trie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclam
Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. Classified as an aza-crown ether, it is a white solid that is soluble in water. As a macrocyclic ligand, it binds strongly to many transition metal cations. The compound was first prepared by the reaction of 1,3-dibromopropane and ethylenediamine. The compound features four secondary amines. Its complexes therefore can exist as several diastereomers, depending on the relative orientation of the N–H centres. Its complexes feature alternating five- and six-membered chelate rings. The closely related ligand cyclen ((CH2CH2NH)4) forms only five-membered C2N2M chelate rings and tends not to form square-planar complexes. ''N''-Alkyl derivatives Metal-cyclam complexes are prone to oxidative degradation, which is initiated by deprotonation of the secondary amine. This flaw led to the development of cyclam derivatives wherein the NH centres are replaced by tertiary amines. F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclen
Cyclen (1,4,7,10-tetraazacyclododecane) is a aza-crown ether with the formula (CH2CH2NH)4. It is a white solid. Synthesis Some syntheses exploit the Thorpe-Ingold effect to facilitate ring-formation. Illustrative is the reaction of the deprotonated tosylamides with ditosylates: :TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4 The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine. High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL. : In one study cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


Cl2.png)