|
Tetramethylammonium Chloride
Tetramethylammonium chloride is one of the simplest quaternary ammonium salts, with four methyl groups tetrahedrally attached to the central N. The chemical formula (CH3)4N+Cl− is often abbreviated further as Me4N+Cl−. It is a hygroscopic colourless solid that is soluble in water and polar organic solvents. Tetramethylammonium chloride is a major industrial chemical, being used widely as a chemical reagent and also as a low-residue bactericide in such processes as hydrofracking. In the laboratory, it has fewer synthetic chemical applications than quaternary ammonium salts containing longer N-alkyl substituents, which are used extensively as phase-transfer catalysts. Preparation and laboratory uses Tetramethylammonium chloride is efficiently produced by the reaction of trimethylamine and methyl chloride. :N(CH3)3 + CH3Cl → N(CH3)4+Cl− It is produced by the alkylation of ammonium chloride with dimethyl carbonate in the presence of an ionic liquid catalyst. Except un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylammonium Hydroxide
Tetramethylammonium hydroxide (TMAH or TMAOH) is a quaternary ammonium salt with molecular formula N(CH3)4+ OH−. It is commonly encountered in form of concentrated solutions in water or methanol. TMAH in solid state and its aqueous solutions are all colorless, but may be yellowish if impure. Although TMAH has virtually no odor when pure, samples often have a strong fishy smell due to presence of trimethylamine which is a common impurity. TMAH has several diverse industrial and research applications. Chemical properties Structure TMAH is most commonly encountered as an aqueous solution, in concentrations from ~2–25%, and less frequently as solutions in methanol. These solutions are identified by CAS numbe75-59-2 Several hydrates such as N(CH3)4OH·xH2O. have been crystallized. These salts contain well separated Me4N+ cations and hydroxide anions ( Me is an abbreviation of methyl group). The hydroxide groups are linked by hydrogen bonds to the water of crystallization ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Carbonate
Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the US. Dimethyl carbonate is often considered to be a green reagent. Production World production in 1997 was estimated at 1000 barrels a day. Production of dimethyl carbonate worldwide is limited to Asia, the Middle East, and Europe. Dimethyl carbonate is traditionally prepared by the reaction of phosgene and methanol. Methyl chloroformate is produced as an intermediate: : COCl2 + CH3OH → CH3OCOCl + HCl : CH3OCOCl + CH3OH → CH3OCO2CH3 + HCl This synthesis route has been largely replaced by oxidative carbonylation. In this process, carbon monoxide and an oxidizer provide the equivalent of CO2+: :: CO + 1/2 O2 + 2 CH3OH → (CH3O)2CO + H2O It can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylammonium Hydroxide
Tetramethylammonium hydroxide (TMAH or TMAOH) is a quaternary ammonium salt with molecular formula N(CH3)4+ OH−. It is commonly encountered in form of concentrated solutions in water or methanol. TMAH in solid state and its aqueous solutions are all colorless, but may be yellowish if impure. Although TMAH has virtually no odor when pure, samples often have a strong fishy smell due to presence of trimethylamine which is a common impurity. TMAH has several diverse industrial and research applications. Chemical properties Structure TMAH is most commonly encountered as an aqueous solution, in concentrations from ~2–25%, and less frequently as solutions in methanol. These solutions are identified by CAS numbe75-59-2 Several hydrates such as N(CH3)4OH·xH2O. have been crystallized. These salts contain well separated Me4N+ cations and hydroxide anions ( Me is an abbreviation of methyl group). The hydroxide groups are linked by hydrogen bonds to the water of crystallization ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium Chloride
Tetraethylammonium chloride (TEAC) is a quaternary ammonium compound with the chemical formula (C2H5)4N+Cl−, sometimes written as Et4N+Cl−. In appearance, it is a hygroscopic, colorless, crystalline solid. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis. Preparation and structure TEAC is produced by alkylation of triethylamine with ethyl chloride. TEAC exists as either of two stable hydrates, the monohydrate and tetrahydrate. The crystal structure of TEAC.H2O has been determined, as has that of the tetrahydrate, TEAC.4H2O. Details for the preparation of large, prismatic crystals of TEAC.H2O are given by Harmon and Gabriele, who carried out IR-spectroscopic studies on this and related compounds. These researchers have also pointed out that, although freshly-purified TEAC.H2O is free of triethylamine hydrochloride, small quantities of this compound form on heating of TEAC as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
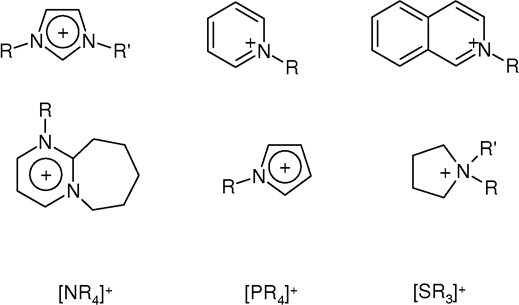
Quaternary Ammonium Cation
In chemistry, quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure , R being an alkyl group or an aryl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis Quaternary ammonium compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base Pairs
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA and RNA. Dictated by specific hydrogen bonding patterns, "Watson–Crick" (or "Watson–Crick–Franklin") base pairs (guanine–cytosine and adenine–thymine) allow the DNA helix to maintain a regular helical structure that is subtly dependent on its nucleotide sequence. The complementary nature of this based-paired structure provides a redundant copy of the genetic information encoded within each strand of DNA. The regular structure and data redundancy provided by the DNA double helix make DNA well suited to the storage of genetic information, while base-pairing between DNA and incoming nucleotides provides the mechanism through which DNA polymerase replicates DNA and RNA polymerase transcribes DNA into RNA. Many DNA-binding proteins ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Concentration
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solution. In chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol/ dm3 in SI unit. A solution with a concentration of 1 mol/L is said to be 1 molar, commonly designated as 1 M. Definition Molar concentration or molarity is most commonly expressed in units of moles of solute per litre of solution. For use in broader applications, it is defined as amount of substance of solute per unit volume of solution, or per unit volume available to the species, represented by lowercase c: :c = \frac = \frac = \frac. Here, n is the amount of the solute in moles, N is the number of constituent particles present in volume V (in litres) of the solution, and N_\text is the Av ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerase Chain Reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) to a large enough amount to study in detail. PCR was invented in 1983 by the American biochemist Kary Mullis at Cetus Corporation; Mullis and biochemist Michael Smith (chemist), Michael Smith, who had developed other essential ways of manipulating DNA, were jointly awarded the Nobel Prize in Chemistry in 1993. PCR is fundamental to many of the procedures used in genetic testing and research, including analysis of Ancient DNA, ancient samples of DNA and identification of infectious agents. Using PCR, copies of very small amounts of DNA sequences are exponentially amplified in a series of cycles of temperature changes. PCR is now a common and often indispensable technique used in medical laboratory research for a broad variety of applications ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitation (chemistry)
In an aqueous solution, precipitation is the process of transforming a dissolved chemical substance, substance into an insoluble solid from a Supersaturated solution, super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the ''precipitant''. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'supernate' or 'supernatant'. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (''e.g.'', metallurgy and alloys) when solid impurities Segregation (materials science), segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium
Tetraethylammonium (TEA), () or (Et4N+) is a quaternary ammonium cation consisting of four ethyl groups attached to a central nitrogen atom, and is positively charged. It is a counterion used in the research laboratory to prepare lipophilic salts of inorganic anions. It is used similarly to tetrabutylammonium, the difference being that its salts are less lipophilic and more easily crystallized. Preparation The chloride salt is prepared by the reaction of triethylamine and an ethyl halide: :Et3N + EtX → Et4N+X− This method works well for the preparation of tetraethylammonium iodide (where X = I). Most tetraethylammonium salts are prepared by salt metathesis reactions. For example, the synthesis of tetraethylammonium perchlorate, a salt that has been useful as a supporting electrolyte for polarographic studies in non-aqueous solvents, is carried out by mixing the water-soluble salts tetraethylammonium bromide and sodium perchlorate in water, from which the water-insoluble tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)