|
Tartronic Acid Semialdehyde
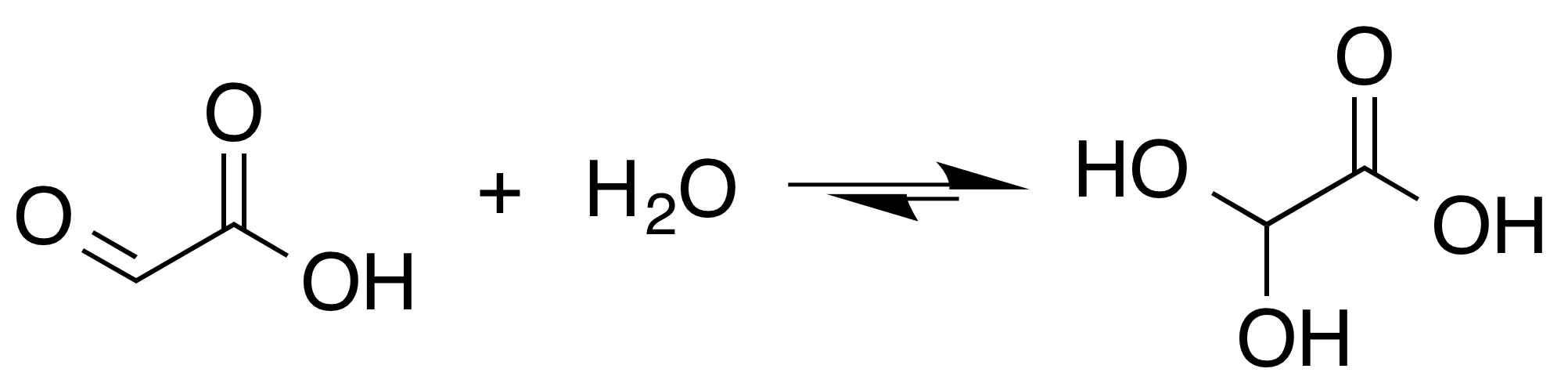
Tartronic acid semialdehyde is the organic compound with the formula OCHCH(OH)CO2H. The molecule has three functional groups, aldehyde, alcohol, and carboxylic acid. A white solid, it occurs naturally. A near neutral pH, it exists as the hydrated carboxylate (HO)2CHCH(OH)CO2−, which is referred to as tartronate semialdehyde. Tartronate semialdehyde is produced and consumed on a prodigious scale as an intermediate in photorespiration, an undesirable side reaction that competes with photosynthesis Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i .... It is produced biologically by the condensation of two equivalents of glyoxalate: :2{{nbspOC(H)CO2H → OC(H)CH(OH)CO2H + CO2 This condensation is catalyzed by tartronate-semialdehyde synthase. References Alpha hydroxy aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photorespiration
Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. The desired reaction is the addition of carbon dioxide to RuBP (carboxylation), a key step in the Calvin–Benson cycle, but approximately 25% of reactions by RuBisCO instead add oxygen to RuBP ( oxygenation), creating a product that cannot be used within the Calvin–Benson cycle. This process lowers the efficiency of photosynthesis, potentially lowering photosynthetic output by 25% in plants. Photorespiration involves a complex network of enzyme reactions that exchange metabolites between chloroplasts, leaf peroxisomes and mitochondria. The oxygenation reaction of RuBisCO is a wasteful process because 3-phosphoglycerate is created at a lower rate and higher metabolic cost compared with RuBP carboxylase activity. While photorespiratory carbon cycling resu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water – hence the name ''photosynthesis'', from the Greek ''phōs'' (), "light", and ''synthesis'' (), "putting together". Most plants, algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth. Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centers that contain green chlorophyll (and other colored) pigments/chromoph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structure has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tartronate-semialdehyde Synthase
The enzyme tartronate-semialdehyde synthase () catalyzes the chemical reaction :2 glyoxylate \rightleftharpoons tartronate semialdehyde + CO2 This enzyme belongs to the family of lyases, specifically the carboxy-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is glyoxylate carboxy-lyase (dimerizing tartronate-semialdehyde-forming). Other names in common use include tartronate semialdehyde carboxylase, glyoxylate carbo-ligase, glyoxylic carbo-ligase, hydroxymalonic semialdehyde carboxylase, tartronic semialdehyde carboxylase, glyoxalate carboligase, and glyoxylate carboxy-lyase (dimerizing). This enzyme participates in glyoxylate and dicarboxylate metabolism. It has 2 cofactors: FAD, and Thiamin diphosphate Thiamine pyrophosphate (TPP or ThPP), or thiamine diphosphate (ThDP), or cocarboxylase is a thiamine (vitamin B1) derivative which is produced by the enzyme thiamine diphosphokinase. Thiamine pyrophosphate is a cofactor that is present ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Hydroxy Acids
α-Hydroxy acids, or alpha hydroxy acids (AHAs), are a class of chemical compounds that consist of a carboxylic acid with a hydroxyl group substituent on the adjacent (alpha) carbon. Prominent examples are glycolic acid, lactic acid, mandelic acid and citric acid. Although these compounds are related to the ordinary carboxylic acids and are therefore weak acids, their chemical structure allows for the formation of an internal hydrogen bond between the hydrogen at the hydroxyl group and one of the oxygen atoms of the carboxylic group. The net effect is an increase in acidity. For example, the pKa of lactic acid is 3.86, while that of the unsubstituted propionic acid is 4.87; a full pKa unit difference means that lactic acid is ten times stronger than propionic acid. Industrial applications Feed additives 2-Hydroxy-4-(methylthio)butyric acid is produced commercially as a racemic mixture to substitute for methionine in animal feed. In nature, the same compound is an intermediate in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |