|
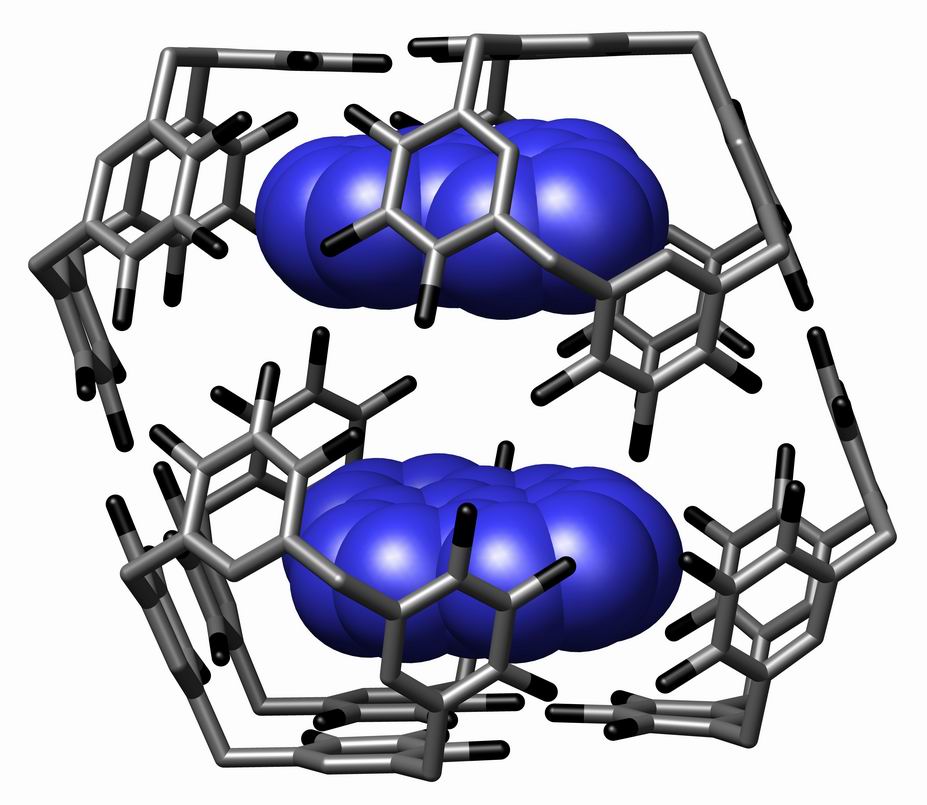
Supramolecule
The term supermolecule (or supramolecule) was introduced by Karl Lothar Wolf ''et al.'' (''Übermoleküle'') in 1937 to describe hydrogen-bonded acetic acid dimers. The study of non-covalent association of complexes of molecules has since developed into the field of supramolecular chemistry. The term supermolecule is sometimes used to describe supramolecular assemblies, which are complexes of two or more molecules (often macromolecules) that are not covalently bonded. The term supermolecule is also used in biochemistry to describe complexes of biomolecules, such as peptides and oligonucleotides composed of multiple strands. See also *Macromolecule *Molecular self-assembly * Supramolecular assembly *Supramolecular chemistry Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ... * Van de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid Dimers
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or developmental biology, development. Biomolecules include large macromolecules (or polyelectrolytes) such as proteins, carbohydrates, lipids, and nucleic acids, as well as small molecules such as primary metabolites, secondary metabolites and natural products. A more general name for this class of material is biological materials. Biomolecules are an important element of living organisms, those biomolecules are often endogeny (biology), endogenous, produced within the organism but organisms usually need exogeny, exogenous biomolecules, for example certain nutrients, to survive. Biology and its subfields of biochemistry and molecular biology study biomolecules and their organic reaction, reactions. Most biomolecules are organic compounds, and just four chemical element, elements ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, molecular folding, molecular recognition, host–guest chemistry, mechanically-interlocked molecular architectures, and dynamic covalent chemistry. The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Assembly
In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules (e.g., a DNA double helix or an inclusion compound), or a defined number of stoichiometrically interacting molecules within a quaternary complex, it is more often used to denote larger complexes composed of indefinite numbers of molecules that form sphere-, rod-, or sheet-like species. Colloids, liquid crystals, biomolecular condensates, micelles, liposomes and biological membranes are examples of supramolecular assemblies, and their realm of study is known as supramolecular chemistry. The dimensions of supramolecular assemblies can range from nanometers to micrometers. Thus they allow access to nanoscale objects using a bottom-up approach in far fewer steps than a single molecule of similar dimensions. The process by which a supramolecular assembly forms is called molecular self-assembly. Some try to di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Self-assembly
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intramolecular and intermolecular. Commonly, the term ''molecular self-assembly'' refers to the former, while the latter is more commonly called '' folding''. Supramolecular systems Molecular self-assembly is a key concept in supramolecular chemistry. This is because assembly of molecules in such systems is directed through non-covalent interactions (e.g., hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi-stacking interactions, and/or electrostatic) as well as electromagnetic interactions. Common examples include the formation of colloids, biomolecular condensates, micelles, vesicles, liquid crystal phases, and Langmuir monolayers by surfactant molecules. Further examples of supramolecular assemblies demonstrate that a variet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The most common macromolecules in biochemistry are biopolymers (nucleic acids, proteins, and carbohydrates) and large non-polymeric molecules such as lipids, nanogels and macrocycles. Synthetic fibers and experimental materials such as carbon nanotubes are also examples of macromolecules. Definition The term ''macromolecule'' (''macro-'' + ''molecule'') was coined by Nobel laureate Hermann Staudinger in the 1920s, although his first relevant publication on this field only mentions ''high molecular compounds'' (in excess of 1,000 atoms). At that time the term ''polymer'', as introduced by Berzelius in 1832, had a different meaning from that of today: it simply was another form of isomerism for example with benzene and acetylene and had lit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small bits of nucleic acids can be manufactured as single-stranded molecules with any user-specified sequence, and so are vital for artificial gene synthesis, polymerase chain reaction (PCR), DNA sequencing, molecular cloning and as molecular probes. In nature, oligonucleotides are usually found as small RNA molecules that function in the regulation of gene expression (e.g. microRNA), or are degradation intermediates derived from the breakdown of larger nucleic acid molecules. Oligonucleotides are characterized by the sequence of nucleotide residues that make up the entire molecule. The length of the oligonucleotide is usually denoted by " -mer" (from Greek ''meros'', "part"). For example, an oligonucleotide of six nucleotides (nt) is a hexamer, while one of 25 nt w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research. Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells, Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function.Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenomena.Ast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karl Lothar Wolf
Karl may refer to: People * Karl (given name), including a list of people and characters with the name * Karl der Große, commonly known in English as Charlemagne * Karl Marx, German philosopher and political writer * Karl of Austria, last Austrian Emperor * Karl (footballer) (born 1993), Karl Cachoeira Della Vedova Júnior, Brazilian footballer In myth * Karl (mythology), in Norse mythology, a son of Rig and considered the progenitor of peasants (churl) * ''Karl'', giant in Icelandic myth, associated with Drangey island Vehicles * Opel Karl, a car * ST ''Karl'', Swedish tugboat requisitioned during the Second World War as ST ''Empire Henchman'' Other uses * Karl, Germany, municipality in Rhineland-Palatinate, Germany * '' Karl-Gerät'', AKA Mörser Karl, 600mm German mortar used in the Second World War * KARL project, an open source knowledge management system * Korean Amateur Radio League, a national non-profit organization for amateur radio enthusiasts in South Korea * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The most common macromolecules in biochemistry are biopolymers (nucleic acids, proteins, and carbohydrates) and large non-polymeric molecules such as lipids, nanogels and macrocycles. Synthetic fibers and experimental materials such as carbon nanotubes are also examples of macromolecules. Definition The term ''macromolecule'' (''macro-'' + ''molecule'') was coined by Nobel laureate Hermann Staudinger in the 1920s, although his first relevant publication on this field only mentions ''high molecular compounds'' (in excess of 1,000 atoms). At that time the term ''polymer'', as introduced by Berzelius in 1832, had a different meaning from that of today: it simply was another form of isomerism for example with benzene and acetylene and had lit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |