|
Sodium Monofluorophosphate
Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, MFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann "Phosphoric Acid and Phosphates" in ''Ullmann’s Encyclopedia of Industrial Chemistry'' 2008, Wiley-VCH, Weinheim. Uses MFP is best known as an ingredient in some toothpastes.Wolfgang Weinert "Oral Hygiene Products" in ''Ullmann’s Encyclopedia of Industrial Chemistry'' 2000, Wiley-VCH, Weinheim. It functions as a source of fluoride via the following hydrolysis reaction: :PO3F2− + OH− → HPO42− + F− Fluoride protects tooth enamel from attack by bacteria that cause dental caries (cavities). Although developed by a chemist at Procter and Gamble, its use in toothpaste (Colgate toothpaste and Ultra Brite) was patented by Colgate-Palmolive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calgon
Calgon is an American brand of bath and beauty products, owned by PDC Brands. Early history The original product consisted of powdered sodium hexametaphosphate (amorphous sodium polyphosphate), which in water would complex with ambient calcium ions and certain other cations, preventing formation of unwanted salts and interference by those cations with the actions of soap or other detergents. Its name was a portmanteau derived from the phrase "calcium gone".Walker, Andrea K. (June 22, 2010)"Calgon, take me away...again" ''The Baltimore Sun''. Originally promoted for general use in bathing and cleaning, it gave rise to derivative products which have diverged from the original composition. Today, Calgon water softener contains the active ingredient sodium citrate and the now discontinued powder used zeolite and polycarboxylate, all of which are less problematic in wastewater treatment than phosphates. Companies The brands have their origin in Calgon, Inc. of Pittsburgh, Penn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and deterge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disodium Phosphate
Disodium phosphate (DSP), or disodium hydrogen phosphate, or sodium phosphate dibasic, is the inorganic compound with the formula Na2HPO4. It is one of several sodium phosphates. The salt is known in anhydrous form as well as forms with 2, 7, 8, and 12 hydrates. All are water-soluble white powders; the anhydrous salt being hygroscopic. The pH of disodium hydrogen phosphate water solution is between 8.0 and 11.0, meaning it is moderately basic: :HPO42− + H2O H2PO4− + OH− Production and reactions It can be generated by neutralization of phosphoric acid with sodium hydroxide: :H3PO4 + 2 NaOH → Na2HPO4 + 2 H2O Industrially It is prepared in a two-step process by treating dicalcium phosphate with sodium bisulfate, which precipitates calcium sulfate:Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann "Phosphoric Acid and Phosphates" in ''Ullmann’s Encyclopedia of Industrial Chemistry'' 2008, Wiley-VCH, Weinhei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrasodium Pyrophosphate
Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is an inorganic compound with the chemical formula, formula Na4P2O7. As a salt, it is a white, water-soluble solid. It is composed of pyrophosphate anion and sodium ions. Toxicity is approximately twice that of table salt when ingested orally.Handbook of food toxicology', S. S. Deshpande, page 260 Also known is the decahydrate Na4P2O710(H2O). D.L. Perry S.L. Phillips (1995) ''Handbook of inorganic compounds'' CRC Press Use Tetrasodium pyrophosphate is used as a buffering agent, an emulsifier, a dispersing agent, and a thickening agent, and is often used as a food additive. Common foods containing tetrasodium pyrophosphate include chicken nuggets, marshmallows, pudding, crab meat, imitation crab, canned tuna, and soy-based meat alternatives and cat foods and cat treats where it is used as a palatability enhancer. In toothpaste and dental floss, tetrasodium pyrophosphate acts as a Calculus ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metaphosphate
A metaphosphate ion is an oxyanion that has the empirical formula . It was first postulated in 1955 but was not observed until 1979, when it was detected by mass spectrometry. Metaphosphate is an intermediate in the hydrolysis of phosphate esters but it is difficult to isolate, as it readily hydrolyses to from a dihydrogen phosphate ion () and tends to self-react in the absence of water to form rings or infinite chains: These species are also called metaphosphates and are generally stable, with some such as sodium trimetaphosphate being produced on an industrial scale. Metaphosphates can be used as an alternative of white phosphorus in organic syntheses. See also *Sodium trimetaphosphate *Sodium hexametaphosphate Sodium hexametaphosphate (SHMP) is a salt of composition Na6 PO3)6 Sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: NaPO3), of which the hexamer is one, and is usually the compound referred to by t ... References {{ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorapatite
Fluorapatite, often with the alternate spelling of fluoroapatite, is a phosphate mineral with the formula Ca5(PO4)3F (calcium fluorophosphate). Fluorapatite is a hard crystalline solid. Although samples can have various color (green, brown, blue, yellow, violet, or colorless), the pure mineral is colorless, as expected for a material lacking transition metals. Along with hydroxylapatite, it can be a component of tooth enamel, but for industrial use both minerals are mined in the form of phosphate rock, whose usual mineral composition is primarily fluorapatite but often with significant amounts of the other. Fluorapatite crystallizes in a hexagonal crystal system. It is often combined as a solid solution with hydroxylapatite (Ca5(PO4)3OH or Ca10(PO4)6(OH)2) in biological matrices. Chlorapatite (Ca5(PO4)3Cl) is another related structure. Industrially, the mineral is an important source of both phosphoric and hydrofluoric acids. Fluorapatite as a mineral is the most common p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyapatite
Hydroxyapatite, also called hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but it is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. Hydroxyapatite is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride, chloride or carbonate, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis. Up to 50% by volume and 70% by weight of human bone is a modified form of hydroxyapatite, known as bone mineral. Carbonated calcium-deficient hydroxyapatite is the main mineral of which dental enamel and dentin are composed. Hydroxyapatite crystals are also found in pathological calcifications such as those found in breast tumors, as w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
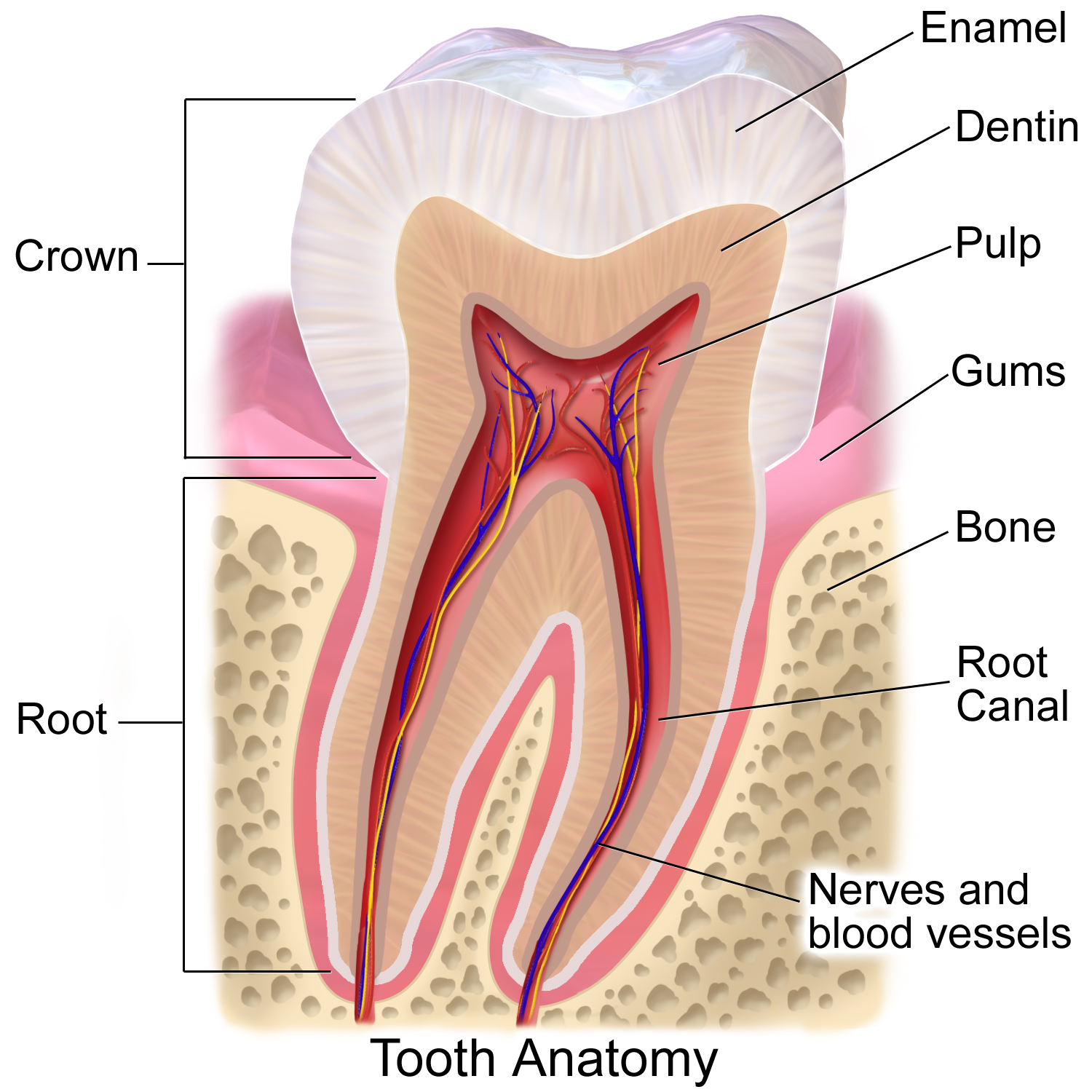
Tooth Enamel
Tooth enamel is one of the four major Tissue (biology), tissues that make up the tooth in humans and many other animals, including some species of fish. It makes up the normally visible part of the tooth, covering the Crown (tooth), crown. The other major tissues are dentin, cementum, and Pulp (tooth), dental pulp. It is a very hard, white to off-white, highly mineralised substance that acts as a barrier to protect the tooth but can become susceptible to degradation, especially by acids from food and drink. Calcium hardens the tooth enamel. In rare circumstances enamel fails to form, leaving the underlying dentin exposed on the surface. Features Enamel is the hardest substance in the human body and contains the highest percentage of minerals (at 96%),Ross ''et al.'', p. 485 with water and organic material composing the rest.Ten Cate's Oral Histology, Nancy, Elsevier, pp. 70–94 The primary mineral is hydroxyapatite, which is a crystalline calcium phosphate. Enamel is formed o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars, and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as glycerol and sugar alcohols, may have a sweet taste, but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural sources of simple sugars. Suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc). Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules: the linear and helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the energy reserve of animals, is a more highly branched version of amylopectin. In industry, starch is often converted into sugars, for example by malting. These sugars may be fermented to produce ethanol in the manufacture of beer, whisky and biofuel. In addition, sugars produced from processed starch are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |