|
Fluorapatite
Fluorapatite, often with the alternate spelling of fluoroapatite, is a phosphate mineral with the formula Ca5(PO4)3F (calcium fluorophosphate). Fluorapatite is a hard crystalline solid. Although samples can have various color (green, brown, blue, yellow, violet, or colorless), the pure mineral is colorless, as expected for a material lacking transition metals. Along with hydroxylapatite, it can be a component of tooth enamel, but for industrial use both minerals are mined in the form of phosphate rock, whose usual mineral composition is primarily fluorapatite but often with significant amounts of the other. Fluorapatite crystallizes in a hexagonal crystal system. It is often combined as a solid solution with hydroxylapatite (Ca5(PO4)3OH or Ca10(PO4)6(OH)2) in biological matrices. Chlorapatite (Ca5(PO4)3Cl) is another related structure. Industrially, the mineral is an important source of both phosphoric and hydrofluoric acids. Fluorapatite as a mineral is the most co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. Howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorapatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate Mineral
Phosphate minerals contain the tetrahedrally coordinated phosphate (PO43−) anion along sometimes with arsenate (AsO43−) and vanadate (VO43−) substitutions, and chloride (Cl−), fluoride (F−), and hydroxide (OH−) anions that also fit into the crystal structure. The phosphate class of minerals is a large and diverse group, however, only a few species are relatively common. Applications Phosphate rock has high concentration of phosphate minerals, most commonly of the apatite group. It is the major resource mined to produce phosphate fertilizers for the agriculture sector. Phosphate is also used in animal feed supplements, food preservatives, anti-corrosion agents, cosmetics, fungicides, ceramics, water treatment and metallurgy. The largest use of minerals mined for their phosphate content is the production of fertilizer. Phosphate minerals are often used for control of rust and prevention of corrosion on ferrous materials applied with electrochemical conver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylapatite
Hydroxyapatite, also called hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but it is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. Hydroxyapatite is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride, chloride or carbonate, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis. Up to 50% by volume and 70% by weight of human bone is a modified form of hydroxyapatite, known as bone mineral. Carbonated calcium-deficient hydroxyapatite is the main mineral of which dental enamel and dentin are composed. Hydroxyapatite crystals are also found in pathological calcifications such as those found in breast tumors, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Monofluorophosphate
Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, MFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann "Phosphoric Acid and Phosphates" in ''Ullmann’s Encyclopedia of Industrial Chemistry'' 2008, Wiley-VCH, Weinheim. Uses MFP is best known as an ingredient in some toothpastes.Wolfgang Weinert "Oral Hygiene Products" in ''Ullmann’s Encyclopedia of Industrial Chemistry'' 2000, Wiley-VCH, Weinheim. It functions as a source of fluoride via the following hydrolysis reaction: :PO3F2− + OH− → HPO42− + F− Fluoride protects tooth enamel from attack by bacteria that cause dental caries (cavities). Although developed by a chemist at Procter and Gamble, its use in toothpaste ( Colgate toothpaste and Ultra Brite) was patented by Colgate-Palmol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate Mineral
Phosphate minerals contain the tetrahedrally coordinated phosphate (PO43−) anion along sometimes with arsenate (AsO43−) and vanadate (VO43−) substitutions, and chloride (Cl−), fluoride (F−), and hydroxide (OH−) anions that also fit into the crystal structure. The phosphate class of minerals is a large and diverse group, however, only a few species are relatively common. Applications Phosphate rock has high concentration of phosphate minerals, most commonly of the apatite group. It is the major resource mined to produce phosphate fertilizers for the agriculture sector. Phosphate is also used in animal feed supplements, food preservatives, anti-corrosion agents, cosmetics, fungicides, ceramics, water treatment and metallurgy. The largest use of minerals mined for their phosphate content is the production of fertilizer. Phosphate minerals are often used for control of rust and prevention of corrosion on ferrous materials applied with electrochemical conver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and Group 12 element, group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and Surface prope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptides, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Phosphate
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white solids of nutritious value and are found in many living organisms, e.g., bone mineral and tooth enamel. In milk, it exists in a colloidal form in micelles bound to casein protein with magnesium, zinc, and citrate–collectively referred to as colloidal calcium phosphate (CCP). Various calcium phosphate minerals are used in the production of phosphoric acid and fertilizers. Overuse of certain forms of calcium phosphate can lead to nutrient-containing surface runoff and subsequent adverse effects upon receiving waters such as algal blooms and eutrophication (over-enrichment with nutrients and minerals). Orthophosphates, di- and monohydrogen phosphates These materials contain Ca2+ combined with , , or : * Monocalcium phosphate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
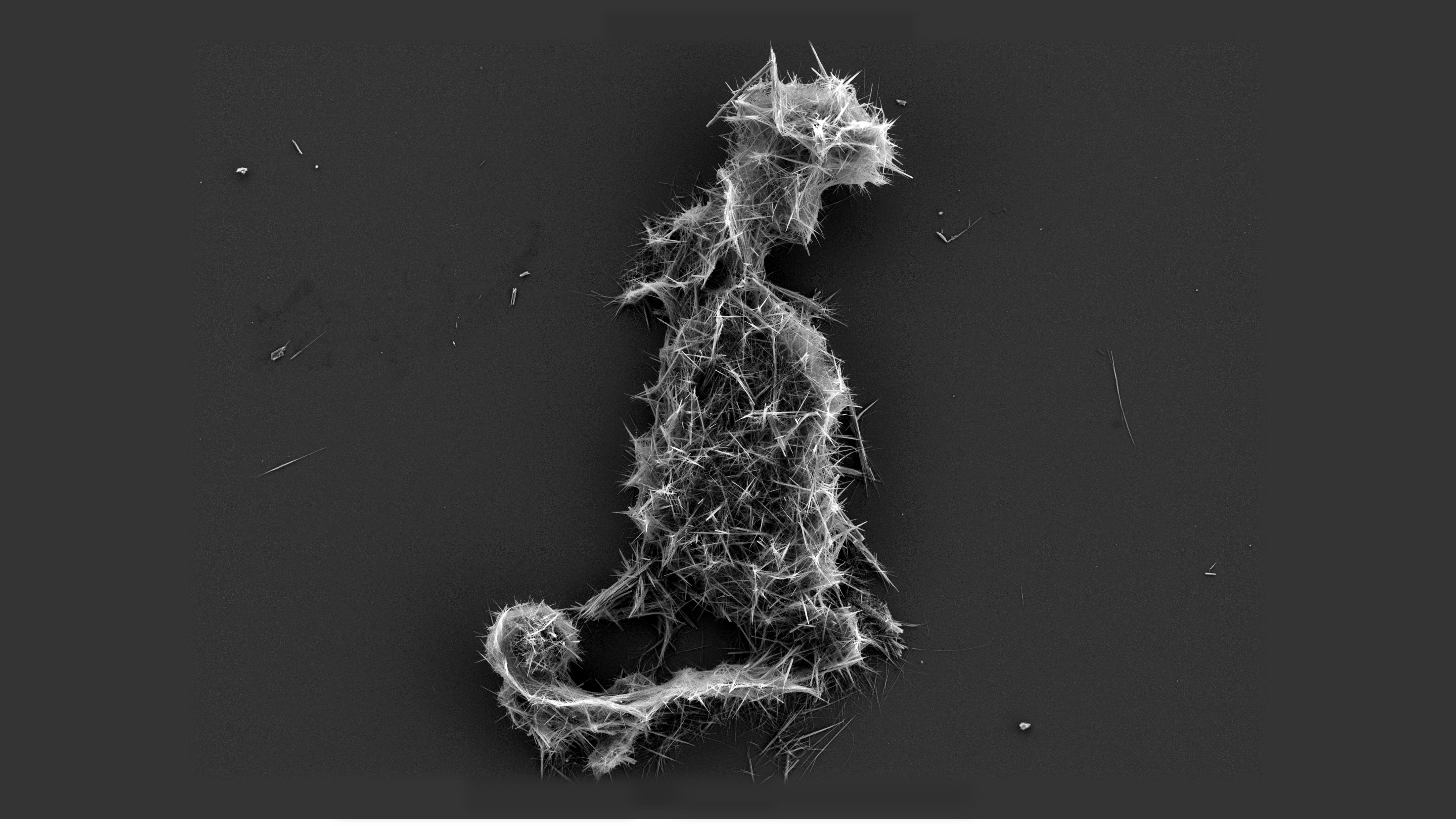
Thin Section Microscopy Siilinjärvi R301 6170 Apatite
Thin may refer to: * a lean body shape. ''(See also: emaciation, underweight)'' * ''Thin'' (film), a 2006 HBO documentary about eating disorders * Paper Thin (other), referring to multiple songs * Thin (web server), a Ruby web-server based on Mongrel *Thin (name) See also * * * Thin client, a computer in a client-server architecture network. * Thin film, a material layer of about 1 μm thickness. * Thin-film deposition, any technique for depositing a thin film of material onto a substrate or onto previously deposited layers * Thin film memory, high-speed variation of core memory developed by Sperry Rand in a government-funded research project * Thin-film optics, the branch of optics that deals with very thin structured layers of different materials * Thin layer chromatography (TLC), a chromatography technique used in chemistry to separate chemical compounds * Thin layers (oceanography), congregations of phytoplankton and zooplankton in the water column * Thin len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptococcus Mutans
''Streptococcus mutans'' is a facultatively anaerobic, gram-positive coccus (round bacterium) commonly found in the human oral cavity and is a significant contributor to tooth decay. It is part of the "streptococci" (plural, non-italic lowercase), an informal general name for all species in the genus ''Streptococcus''. The microbe was first described by James Kilian Clarke in 1924. This bacterium, along with the closely related species '' Streptococcus sobrinus'', can cohabit the mouth: Both contribute to oral disease, and the expense of differentiating them in laboratory testing is often not clinically necessary. Therefore, for clinical purposes they are often considered together as a group, called the mutans streptococci (plural, non-italic due to its being an informal group name). This grouping of similar bacteria with similar tropism can also be seen in the viridans streptococci, another group of ''Streptococcus'' species. Ecology ''S. mutans'' is naturally present in the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |