|
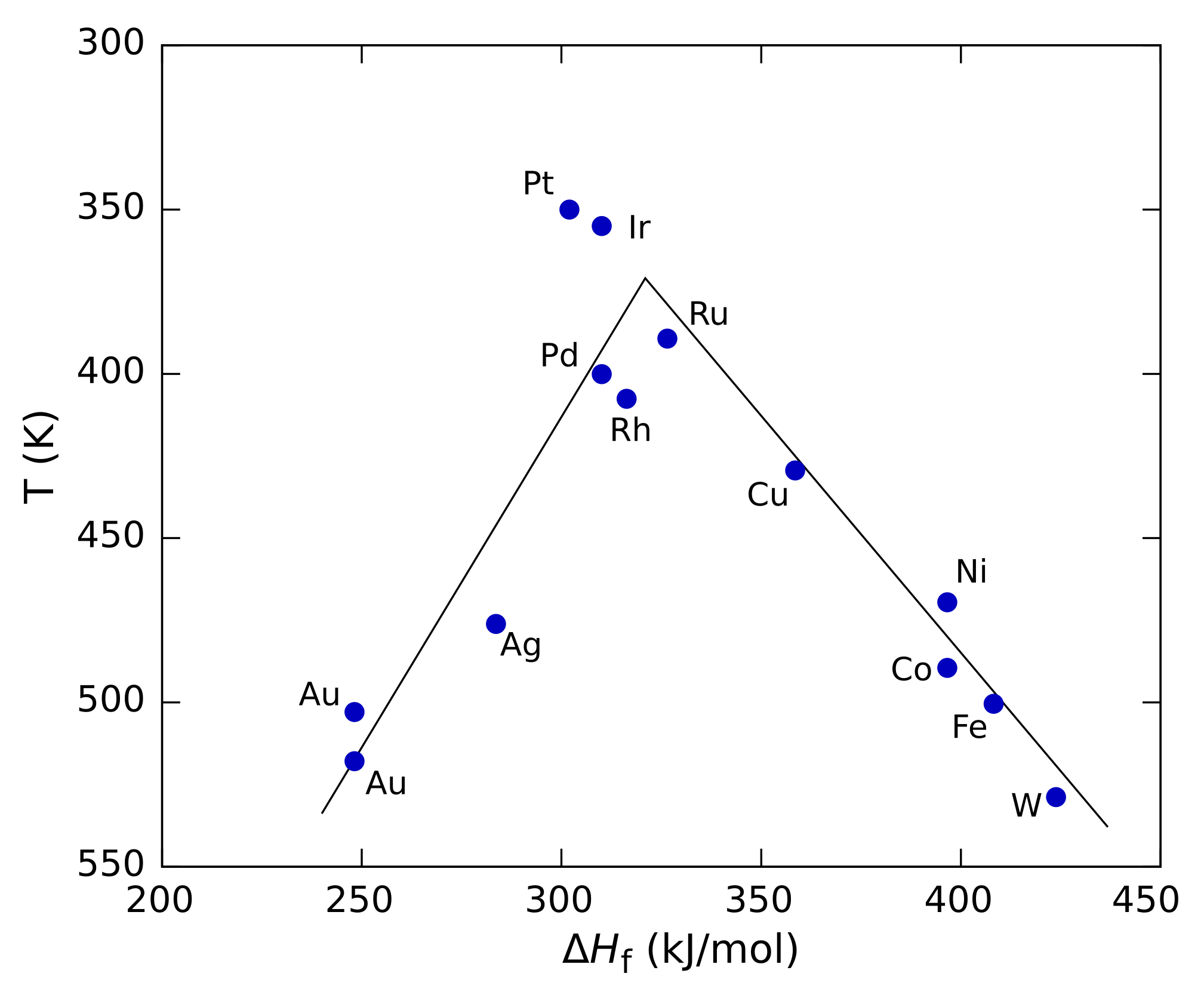
Sabatier Principle
In chemistry, the Sabatier principle is a qualitative concept in heterogeneous catalysis named after the French chemist Paul Sabatier. It states that the interactions between the catalyst and the reactants should be "just right"; that is, neither too strong nor too weak. If the interaction is too weak, the molecule will fail to bind to the catalyst and no reaction will take place. On the other hand, if the interaction is too strong, the product fails to dissociate. The principle can be shown graphically by plotting the reaction rate against a property such as the heat of adsorption of the reactant by the catalyst. Such plots pass through a maximum, looking roughly like a triangle or an inverted parabola, and are called volcano plots because of their shape. Analogous three-dimensional plots can also be built against two different properties, such as the heats of adsorption of the two reactants for a two-component reaction. In that case the plot is generally shown as a contour p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Volcano Plot
Volcano plot may refer to: * Sabatier principle – a concept in chemical catalysis that relates the optimal concentrations of catalysts and substrates * Volcano plot (statistics) – a type of graph used to relate fold-change to p-value that is commonly used in genomics Genomics is an interdisciplinary field of molecular biology focusing on the structure, function, evolution, mapping, and editing of genomes. A genome is an organism's complete set of DNA, including all of its genes as well as its hierarchical, ... and other omic experiments involving thousands of data-points {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Parabola
In mathematics, a parabola is a plane curve which is Reflection symmetry, mirror-symmetrical and is approximately U-shaped. It fits several superficially different Mathematics, mathematical descriptions, which can all be proved to define exactly the same curves. One description of a parabola involves a Point (geometry), point (the Focus (geometry), focus) and a Line (geometry), line (the Directrix (conic section), directrix). The focus does not lie on the directrix. The parabola is the locus (mathematics), locus of points in that plane that are equidistant from the directrix and the focus. Another description of a parabola is as a conic section, created from the intersection of a right circular conical surface and a plane (geometry), plane Parallel (geometry), parallel to another plane that is tangential to the conical surface. The graph of a function, graph of a quadratic function y=ax^2+bx+ c (with a\neq 0 ) is a parabola with its axis parallel to the -axis. Conversely, every ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Platinum Group
The platinum-group metals (PGMs) are six noble, precious metallic elements clustered together in the periodic table. These elements are all transition metals in the d-block (groups 8, 9, and 10, periods 5 and 6). The six platinum-group metals are ruthenium, rhodium, palladium, osmium, iridium, and platinum. They have similar physical and chemical properties, and tend to occur together in the same mineral deposits. However, they can be further subdivided into the ''iridium-group platinum-group elements'' (IPGEs: Os, Ir, Ru) and the ''palladium-group platinum-group elements'' (PPGEs: Rh, Pt, Pd) based on their behaviour in geological systems. The three elements above the platinum group in the periodic table (iron, nickel and cobalt) are all ferromagnetic; these, together with the lanthanide element gadolinium (at temperatures below 20 °C), are the only known transition metals that display ferromagnetism near room temperature. History Naturally occurring plati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Rate-determining Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reaction Intermediate
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear in the chemical equation for the overall reaction. For example, consider this hypothetical reaction: :A + B → C + D If this overall reaction comprises two elementary steps thus: :A + B → X :X → C + D then X is a reaction intermediate. The phrase ''reaction intermediate'' is often abbreviated to the single word ''intermediate'', and this is IUPAC's preferred form of the term. But this shorter form has other uses. It often refers to reactive intermediates. It is also used more widely for chemicals such as cumene which are traded within the chemical industry but are not generally of value outside it. IUPAC definition The IUPAC Gold Book defines an ''intermediate'' as a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Heat Of Formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, with all substances in their standard states. The standard pressure value is recommended by IUPAC, although prior to 1982 the value 1.00 atm (101.325 kPa) was used. There is no standard temperature. Its symbol is Δf''H''⦵. The superscript Plimsoll on this symbol indicates that the process has occurred under standard conditions at the specified temperature (usually 25 °C or 298.15 K). Standard states are defined for various types of substances. For a gas, it is the hypothetical state the gas would assume if it obeyed the ideal gas equation at a pressure of 1 bar. For a gaseous or solid solute present in a diluted ideal solution, the standard state is the hypothetical state of concentration of the solute of exactly one mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. They are lustrous metals with good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramagnetic because of their unpaired d electrons, as are many of their compounds. All of the elements that are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Formic Acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. Natural occurrence Formic acid, which has a pungent, penetrating odor, is found naturally in insects, weeds, fruits and vegetables, and forest emissions. It appears in most ants and in stingless bees of the genus '' Oxytrigona''. Wood ants from the genus ''Formica'' can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (''Cerura vinula'') will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (''Urtica dioica''). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21 mg per 100 g), apple (2 mg per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Contour Plot
A contour line (also isoline, isopleth, isoquant or isarithm) of a function of two variables is a curve along which the function has a constant value, so that the curve joins points of equal value. It is a plane section of the three-dimensional graph of the function f(x,y) parallel to the (x,y)-plane. More generally, a contour line for a function of two variables is a curve connecting points where the function has the same particular value. In cartography, a contour line (often just called a "contour") joins points of equal elevation (height) above a given level, such as mean sea level. A contour map is a map illustrated with contour lines, for example a topographic map, which thus shows valleys and hills, and the steepness or gentleness of slopes. The contour interval of a contour map is the difference in elevation between successive contour lines. The gradient of the function is always perpendicular to the contour lines. When the lines are close together the magnitude of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Heat Of Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term ''sorption'' encompasses both adsorption and absorption, and ''desorption'' is the reverse of sorption. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reaction Rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years, but the combustion of cellulose in a fire is a reaction that takes place in fractions of a second. For most reactions, the rate decreases as the reaction proceeds. A reaction's rate can be determined by measuring the changes in concentration over time. Chemical kinetics is the part of physical chemistry that concerns how rates of chemical reactions are measured and predicted, and how reaction-rate data can be used to deduce probable reaction mechanisms. The concepts of chemical kinetics are applied in many disciplines, such as chemical engineering, enzymology and environmental e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |