Sabatier Principle on:
[Wikipedia]
[Google]
[Amazon]
 The Sabatier principle is a qualitative concept in chemical heterogeneous catalysis named after the French chemist Paul Sabatier. It states that the interactions between the catalyst and the
The Sabatier principle is a qualitative concept in chemical heterogeneous catalysis named after the French chemist Paul Sabatier. It states that the interactions between the catalyst and the
 The Sabatier principle is a qualitative concept in chemical heterogeneous catalysis named after the French chemist Paul Sabatier. It states that the interactions between the catalyst and the
The Sabatier principle is a qualitative concept in chemical heterogeneous catalysis named after the French chemist Paul Sabatier. It states that the interactions between the catalyst and the substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
should be "just right"; that is, neither too strong nor too weak. If the interaction is too weak, the molecule will fail to bind to the catalyst and no reaction will take place. On the other hand, if the interaction is too strong, the product fails to dissociate.
The principle can be shown graphically by plotting the reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
against a property such as the heat of adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a Surface science, surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorpti ...
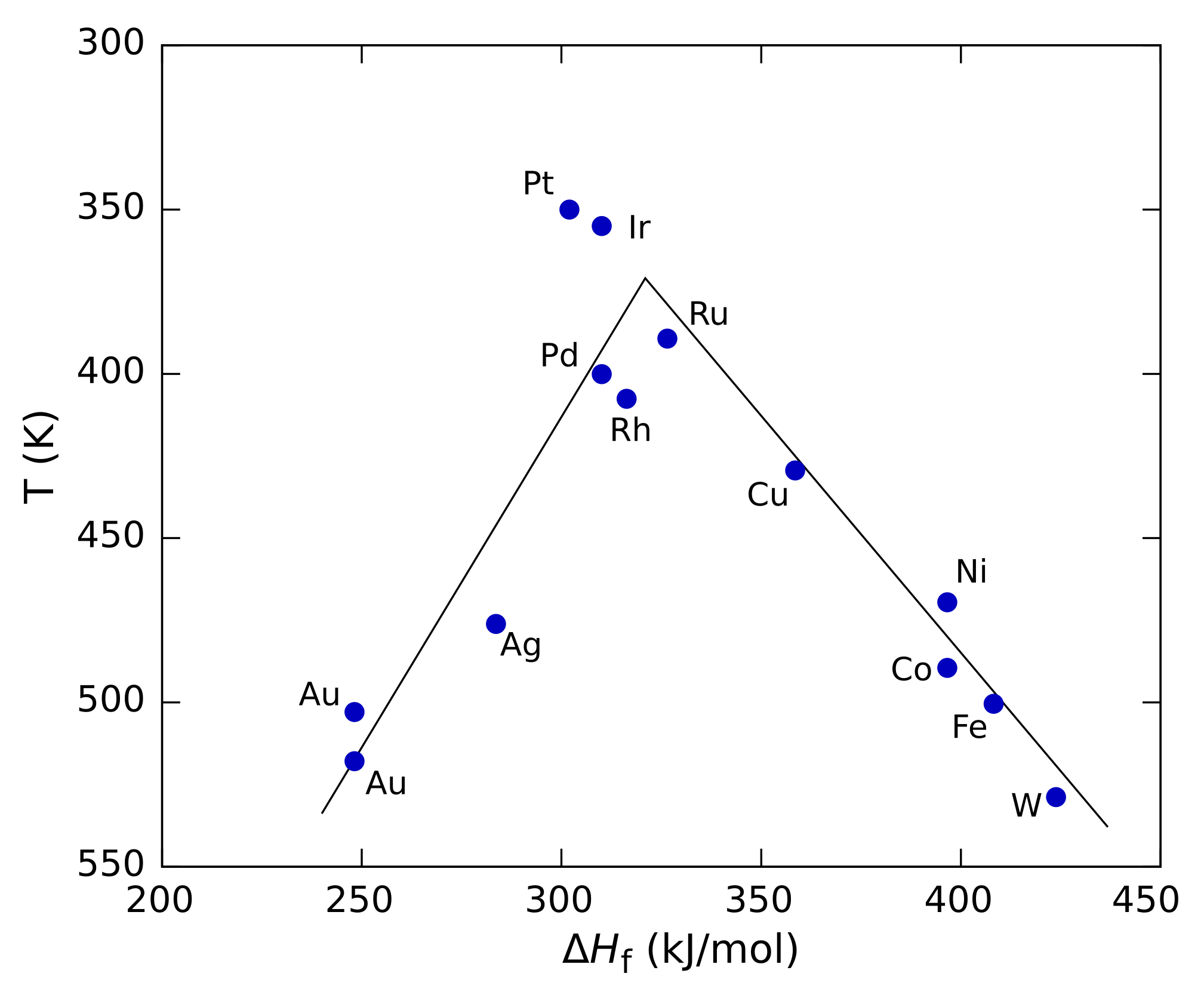
of the reactant by the catalyst. Such plots pass through a maximum, looking roughly like a triangle or an inverted parabola, and are called volcano plots because of their shape. Analogous three-dimensional plots can also be built against two different properties, such as the heats of adsorption of the two reactants for a two-component reaction. In that case the plot is generally shown as a contour plot and is called a volcano surface. Volcano plots were introduced by Balandin.
The figure on the right shows a volcano plot for the decomposition of formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
using different transition metals as catalysts. In this case, the heat of formation (Δ''H''f) of the metal formate salt was used for the x axis because studies showed that the reaction intermediate
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) but is consumed in further reactions in stepwise chemical reactions that contain multiple elementary st ...
was a surface formate. For the y axis, the temperature at which the reaction reaches a specific rate was used (the y axis is plotted in reverse to preserve the conventional "volcano" shape). At low values of Δ''H''f, the reaction is slow (in other words, requires higher temperatures) because the rate of adsorption is slow and rate-limiting
In computer networks, rate limiting is used to control the rate of requests sent or received by a network interface controller. It can be used to prevent DoS attacks and limit web scraping.
Research indicates flooding rates for one zombie mach ...
. At high values of Δ''H''f, desorption becomes the rate-limiting step. The maximum rate, which is observed for the platinum group metals in this case, requires intermediate values of Δ''H''f, with the rate being a combination of the rate of adsorption and the rate of desorption.
References
{{reflist Catalysis