|
SCB-2019
SCB-2019 is a protein subunit COVID-19 vaccine developed by Clover Biopharmaceuticals using an Immunologic adjuvant, adjuvant from Dynavax technologies. Positive results of Phase I trials for the vaccine were published in The Lancet and the vaccine completed enrollment of 29,000 participants in Phase II/III trials in July 2021. In September 2021, SCB-2019 announced Phase III results showing 67% efficacy against all cases of COVID-19 and 79% efficacy against all cases of the Delta variant. Additionally, the vaccine was 84% effective against moderate cases and 100% effective against hospitalization. SCB-2019 is being funded by Coalition for Epidemic Preparedness Innovations, CEPI as part of COVAX and has received advanced purchase orders from GAVI for 400 million doses, with production to begin in 2021. Medical uses The vaccine was administered in 2 doses in 4 weeks. Efficacy In September 2021, SCB-2019 announced Phase III results showing 67.2% efficacy against all cases of C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVAX
COVID-19 Vaccines Global Access, abbreviated as COVAX, is a worldwide initiative aimed at equitable access to COVID-19 vaccines directed by the GAVI vaccine alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO), alongside key delivery partner UNICEF. It is one of the four pillars of the Access to COVID-19 Tools Accelerator, an initiative begun in April 2020 by the WHO, the European Commission, and the government of France as a response to the COVID-19 pandemic. COVAX coordinates international resources to enable low-to-middle-income countries equitable access to COVID-19 tests, therapies, and vaccines. UNICEF is the key delivery partner, leveraging its experience as the largest single vaccine buyer in the world and working on the procurement of COVID-19 vaccine doses, as well as logistics, country readiness and in-country delivery. By 19 October 2020, 184 countries had joined COVAX. COVAX began distributing vaccines in Fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Severe Acute Respiratory Syndrome Coronavirus 2
Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is a strain of coronavirus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the ongoing COVID-19 pandemic. The virus previously had a Novel coronavirus, provisional name, 2019 novel coronavirus (2019-nCoV), and has also been called the human coronavirus 2019 (HCoV-19 or hCoV-19). First identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a public health emergency of international concern on January 30, 2020, and a pandemic on March 11, 2020. SARS‑CoV‑2 is a positive-sense single-stranded RNA virus that is Contagious disease, contagious in humans. SARS‑CoV‑2 is a virus of the species ''severe acute respiratory syndrome–related coronavirus'' (SARSr-CoV), related to the Severe acute respiratory syndrome coronavirus 1, SARS-CoV-1 virus that caused the 2002–2004 SARS outbreak. Despite its close relation to SARS-CoV-1, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
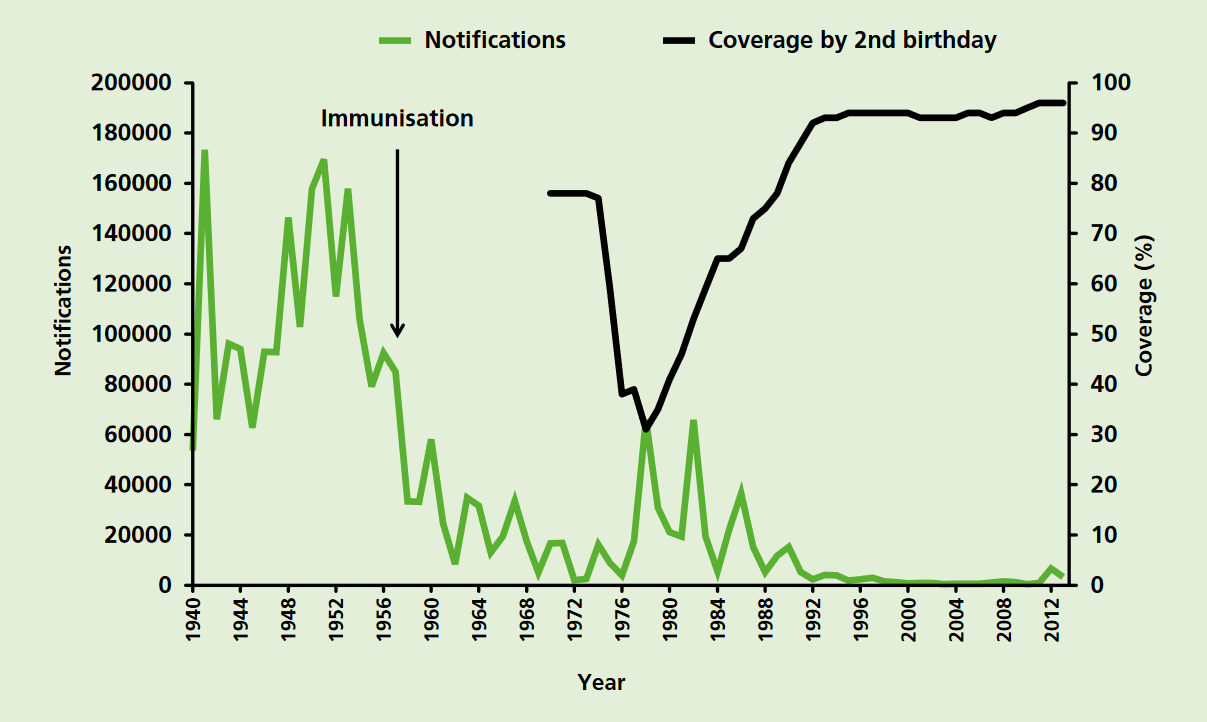
Pertussis Vaccine
Pertussis vaccine is a vaccine that protects against whooping cough (pertussis). There are two main types: whole-cell vaccines and acellular vaccines. The whole-cell vaccine is about 78% effective while the acellular vaccine is 71–85% effective. The effectiveness of the vaccines appears to decrease by between 2 and 10% per year after vaccination with a more rapid decrease with the acellular vaccines. The vaccine is only available in combination with tetanus and diphtheria vaccines. Pertussis vaccine is estimated to have saved over 500,000 lives in 2002. Vaccinating the mother during pregnancy may protect the baby. The World Health Organization and Center for Disease Control and Prevention recommend all children be vaccinated for pertussis and that it be included in Vaccination schedule, routine vaccinations. Three doses starting at six weeks of age are typically recommended in young children. Additional doses may be given to older children and adults. This recommendation inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chinese COVID-19 Vaccines
Chinese can refer to: * Something related to China * Chinese people, people of Chinese nationality, citizenship, and/or ethnicity **''Zhonghua minzu'', the supra-ethnic concept of the Chinese nation ** List of ethnic groups in China, people of various ethnicities in contemporary China ** Han Chinese, the largest ethnic group in the world and the majority ethnic group in Mainland China, Hong Kong, Macau, Taiwan, and Singapore ** Ethnic minorities in China, people of non-Han Chinese ethnicities in modern China ** Ethnic groups in Chinese history, people of various ethnicities in historical China ** Nationals of the People's Republic of China ** Nationals of the Republic of China ** Overseas Chinese, Chinese people residing outside the territories of Mainland China, Hong Kong, Macau, and Taiwan * Sinitic languages, the major branch of the Sino-Tibetan language family ** Chinese language, a group of related languages spoken predominantly in China, sharing a written script (Chinese c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV-2 Delta Variant
The Delta variant (B.1.617.2) was a variant of SARS-CoV-2, the virus that causes COVID-19. It was first detected in India in late 2020. The Delta variant was named on 31 May 2021 and had spread to over 179 countries by 22 November 2021. The World Health Organization (WHO) indicated in June 2021 that the Delta variant was becoming the dominant strain globally. It has mutations in the gene encoding the SARS-CoV-2 coronavirus spike protein, spike protein causing the substitutions T478K, Variants of SARS-CoV-2#P681R, P681R and Variants of SARS-CoV-2#L452R, L452R, which are known to affect transmissibility of the virus as well as whether it can be neutralised by antibodies for previously circulating variants of the COVID-19 virus. In August 2021, Public Health England (PHE) reported secondary attack rate in household contacts of non-travel or unknown cases for Delta to be 10.8% ''vis-à-vis'' 10.2% for the SARS-CoV-2 Alpha variant, Alpha variant; the case fatality rate for those ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SARS-CoV-2 Beta Variant
The Beta variant, (B.1.351), was a variant of SARS-CoV-2, the virus that causes COVID-19. One of several SARS-CoV-2 variants initially believed to be of particular importance, it was first detected in the Nelson Mandela Bay metropolitan area of the Eastern Cape province of South Africa in October 2020, which was reported by the country's health department on 18 December 2020. Phylogeographic analysis suggests this variant emerged in the Nelson Mandela Bay area in July or August 2020. The World Health Organization labelled the variant as Beta variant, not to replace the scientific name but as a name for the public to commonly refer to. The WHO considers it to be a variant of concern no longer in circulation. Names The variant is also known as the ''South African variant''. Mutations File:SARS-CoV-2 Beta variant.svg, Amino acid mutations of SARS-CoV-2 Beta variant plotted on a genome map of SARS-CoV-2 with a focus on Spike. There are three mutations of particular interes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Variant
The Alpha variant (B.1.1.7) was a SARS-CoV-2 variant of concern. It was estimated to be 40–80% more transmissible than the wild-type SARS-CoV-2 (with most estimates occupying the middle to higher end of this range). It was first detected in November 2020 from a sample taken in September in the United Kingdom, and began to spread quickly by mid-December, around the same time as infections surged. This increase is thought to be at least partly because of one or more mutations in the virus' spike protein. The variant was also notable for having more mutations than normally seen. By January 2021, more than half of all genomic sequencing of SARS-CoV-2 was carried out in the UK. This had given rise to questions as to how many other important variants were circulating around the world undetected. On 2 February 2021, Public Health England reported that they had detected " limited number of B.1.1.7 VOC-202012/01 genomes with E484K mutations", which they dubbed Variant of Concer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Journal Of Infectious Diseases
''The Journal of Infectious Diseases'' is a peer-reviewed biweekly medical journal published by Oxford University Press on behalf of the Infectious Diseases Society of America. It covers research on the pathogenesis, medical diagnosis, diagnosis, and therapy, treatment of infectious diseases, on the microbes that cause them, and on immune system disorders. The editor-in-chief is Martin Hirsch, who succeeded Marvin Turck. The journal was established in 1904 and was a quarterly until 1969 when it became a monthly, then in 2001 it began biweekly publication. From 1904 to 2011, the journal was published by the University of Chicago Press. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 5.226. Past editors-in-chief For volumes 1 through 186, the editors-in-chief were: * 1904–1936 Ludvig Hektoen & Edwin O. Jordan (Volumes 1–59) * 1937–1940 Ludvig Hektoen & William H. Tal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perth
Perth is the capital and largest city of the Australian state of Western Australia. It is the fourth most populous city in Australia and Oceania, with a population of 2.1 million (80% of the state) living in Greater Perth in 2020. Perth is part of the South West Land Division of Western Australia, with most of the metropolitan area on the Swan Coastal Plain between the Indian Ocean and the Darling Scarp. The city has expanded outward from the original British settlements on the Swan River, upon which the city's central business district and port of Fremantle are situated. Perth is located on the traditional lands of the Whadjuk Noongar people, where Aboriginal Australians have lived for at least 45,000 years. Captain James Stirling founded Perth in 1829 as the administrative centre of the Swan River Colony. It was named after the city of Perth in Scotland, due to the influence of Stirling's patron Sir George Murray, who had connections with the area. It gained city statu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ZF2001
ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan. ZF2001 employs technology similar to other protein-based vaccines in Phase III trials from Novavax, Vector Institute, and Medicago. ZF2001 was first approved for use in Uzbekistan and later China. Production capacity is expected to be one billion doses a year in China and 200 million in Uzbekistan. By July, 100 million doses had been administered in China and Uzbekistan. Medical uses It is administered in three doses over a period of two months. Efficacy In August 2021, preliminary data from a phase III study with 28,500 participants indicated an overall efficacy of 82% against disease of any severity. Efficacy was 93% against the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abdala (vaccine)
Abdala, technical name CIGB-66, is a COVID-19 vaccine developed by the Center for Genetic Engineering and Biotechnology in Cuba. This candidate, named after a patriotic drama by Cuban independence hero José Martí, is a protein subunit vaccine containing COVID-derived proteins that trigger an immune response. The full results of the clinical trial have not yet been published. This candidate followed a previous one called CIGB-669 (MAMBISA). The vaccine is one of two Cuba-developed COVID-19 vaccines which has passed Phase III trials, and has received emergency authorisation. Medical uses The vaccine was administered in 3 doses spaced 2 weeks apart. Efficacy On 22 June 2021, The official Cuban government sources reported that the results of an initial study by the Cuban Center for Genetic Engineering and Biotechnology involving 48,290 participants found a 92.28% efficacy rate at preventing symptomatic COVID-19. The report included a confidence interval of without a sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)

.jpg)