|
Supramolecular Chirality
In chemistry, the term ''supramolecular chirality'' is used to describe supramolecular assemblies that are non-superposable on their mirror images. Chirality in supramolecular chemistry implies the non-symmetric arrangement of molecular components in a non-covalent assembly. Chirality may arise in a supramolecular system if one of its component is chiral or if achiral components arrange in a non symmetrical way to produce a supermolecule The term supermolecule (or supramolecule) was introduced by Karl Lothar Wolf ''et al.'' (''Übermoleküle'') in 1937 to describe hydrogen-bonded acetic acid dimers. The study of non-covalent association of complexes of molecules has since develope ... that is chiral. References {{chiral synthesis Chirality Stereochemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
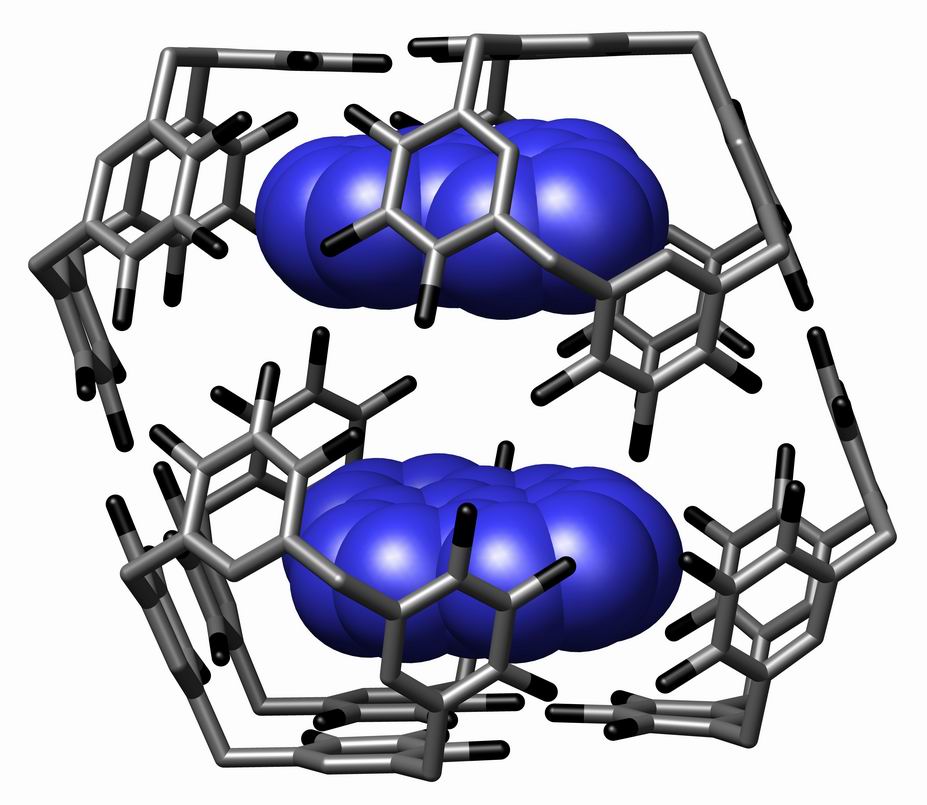
Supramolecular Assembly
In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules (e.g., a DNA double helix or an inclusion compound), or a defined number of stoichiometrically interacting molecules within a quaternary complex, it is more often used to denote larger complexes composed of indefinite numbers of molecules that form sphere-, rod-, or sheet-like species. Colloids, liquid crystals, biomolecular condensates, micelles, liposomes and biological membranes are examples of supramolecular assemblies, and their realm of study is known as supramolecular chemistry. The dimensions of supramolecular assemblies can range from nanometers to micrometers. Thus they allow access to nanoscale objects using a bottom-up approach in far fewer steps than a single molecule of similar dimensions. The process by which a supramolecular assembly forms is called molecular self-assembly. Some try to dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotation (geometry), rotations, translation (geometry), translations, and some Conformational isomerism, conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physics, physical properties, except that they often have opposite optical activity, optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic mixtu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, molecular folding, molecular recognition, host–guest chemistry, mechanically-interlocked molecular architectures, and dynamic covalent chemistry. The st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, molecular folding, molecular recognition, host–guest chemistry, mechanically-interlocked molecular architectures, and dynamic covalent chemistry. The stud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supermolecule
The term supermolecule (or supramolecule) was introduced by Karl Lothar Wolf ''et al.'' (''Übermoleküle'') in 1937 to describe hydrogen-bonded acetic acid dimers. The study of non-covalent association of complexes of molecules has since developed into the field of supramolecular chemistry. The term supermolecule is sometimes used to describe supramolecular assemblies, which are complexes of two or more molecules (often macromolecules) that are not covalently bonded. The term supermolecule is also used in biochemistry to describe complexes of biomolecules, such as peptides and oligonucleotides composed of multiple strands. See also *Macromolecule *Molecular self-assembly *Supramolecular assembly *Supramolecular chemistry *Van der Waals molecule A Van der Waals molecule is a weakly bound complex of atoms or molecules held together by intermolecular attractions such as Van der Waals forces or by hydrogen bonds. The name originated in the beginning of the 1970s when stable mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jean-Marie Lehn
Jean-Marie Lehn (born 30 September 1939) is a French chemist. He received the Nobel Prize in Chemistry together with Donald Cram and Charles Pedersen in 1987 for his synthesis of cryptands. Lehn was an early innovator in the field of supramolecular chemistry, i.e., the chemistry of host–guest molecular assemblies created by intermolecular interactions, and continues to innovate in this field. his group has published 790 peer-reviewed articles in chemistry literature. Biography Early years Lehn was born in Rosheim, Alsace, France to Pierre and Marie Lehn. He is of Alsatian German descent. His father was a baker, but because of his interest in music, he later became the city organist. Lehn also studied music, saying that it became his major interest after science. He has continued to play the organ throughout his professional career as a scientist. His high school studies in Obernai, from 1950 to 1957, included Latin, Greek, German, and English languages, French literatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helvetica Chimica Acta
''Helvetica Chimica Acta'' is a peer-reviewed scientific journal of chemistry established by the Swiss Chemical Society. It is published online by John Wiley & Sons. The journal has a 2020 impact factor of 2.164. History *August 6, 1901: Founding of the Swiss Chemical Society *1911: IUPAC refused SCG as a member, no own journal *September 11, 1917: SCG founded HCA *1917–1948: First editor-in-chief An editor-in-chief (EIC), also known as lead editor or chief editor, is a publication's editorial leader who has final responsibility for its operations and policies. The highest-ranking editor of a publication may also be titled editor, managing ...: Friedrich Fichter (1869–1952) *Spring 1918: Fasciculus I of Volume I of HCA was issued *1948–1971: Emile Cherbuliez (1891–1985) *1970: English allowed as fourth language *1971–1983: Edgardo Giovannini (1909–2004) *1983–2015: M. Volkan Kisakürek *2015-2016: Richard J. Smith *2016–2021: Jeffrey W. Bode and Christophe Copére ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, '' enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |