|
Superactinide
An extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven. , the element with the highest atomic number known is oganesson (''Z'' = 118), which completes the seventh period (row) in the periodic table. All elements in the eighth period and beyond thus remain purely hypothetical. Elements beyond 118 will be placed in additional periods when discovered, laid out (as with the existing periods) to illustrate periodically recurring trends in the properties of the elements concerned. Any additional periods are expected to contain a larger number of elements than the seventh period, as they are calculated to have an additional so-called ''g-block'', containing at least 18 elements with partially filled g- orbitals in each period. An ''eight-period table'' containing this block was suggested by Glenn T. Seaborg in 1969. The first element of the g-block may have atomic number 121, and thus would have the systematic name unb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burkhard Fricke
An extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven. , the element with the highest atomic number known is oganesson (''Z'' = 118), which completes the seventh period (row) in the periodic table. All elements in the eighth period and beyond thus remain purely hypothetical. Elements beyond 118 will be placed in additional periods when discovered, laid out (as with the existing periods) to illustrate periodically recurring trends in the properties of the elements concerned. Any additional periods are expected to contain a larger number of elements than the seventh period, as they are calculated to have an additional so-called ''g-block'', containing at least 18 elements with partially filled g- orbitals in each period. An ''eight-period table'' containing this block was suggested by Glenn T. Seaborg in 1969. The first element of the g-block may have atomic number 121, and thus would have the systematic name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
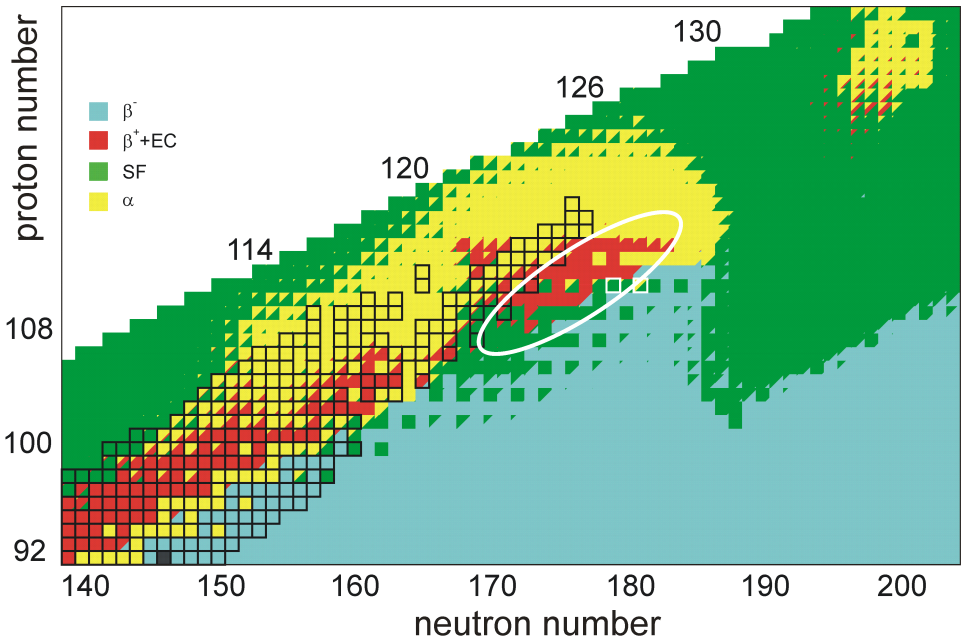
Unbihexium
Unbihexium, also known as element 126 or eka-plutonium, is the hypothetical chemical element with atomic number 126 and placeholder symbol Ubh. ''Unbihexium'' and ''Ubh'' are the temporary IUPAC name and symbol, respectively, until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table, unbihexium is expected to be a g-block superactinide and the eighth element in the 8th period. Unbihexium has attracted attention among nuclear physicists, especially in early predictions targeting properties of superheavy elements, for 126 may be a magic number of protons near the center of an island of stability, leading to longer half-lives, especially for 310Ubh or 354Ubh which may also have magic numbers of neutrons. Early interest in possible increased stability led to the first attempted synthesis of unbihexium in 1971 and searches for it in nature in subsequent years. Despite several reported observations, more recent studies suggest that these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unbiunium
Unbiunium, also known as eka-actinium or simply element 121, is the hypothetical chemical element with symbol Ubu and atomic number 121. ''Unbiunium'' and ''Ubu'' are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be the first of the superactinides, and the third element in the eighth period. It has attracted attention because of some predictions that it may be in the island of stability. It is also likely to be the first of a new g-block of elements. Unbiunium has not yet been synthesized. It is expected to be one of the last few reachable elements with current technology; the limit could be anywhere between element 120 and 124. It will also likely be far more difficult to synthesize than the elements known so far up to 118, and still more difficult than elements 119 and 120. The teams at RIKEN in Japan and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unbibium
Unbibium, also known as element 122 or eka-thorium, is the hypothetical chemical element in the periodic table with the placeholder symbol of Ubb and atomic number 122. ''Unbibium'' and ''Ubb'' are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to follow unbiunium as the second element of the superactinides and the fourth element of the 8th period. Similarly to unbiunium, it is expected to fall within the range of the island of stability, potentially conferring additional stability on some isotopes, especially 306Ubb which is expected to have a magic number of neutrons (184). Despite several attempts, unbibium has not yet been synthesized, nor have any naturally occurring isotopes been found to exist. There are currently no plans to attempt to synthesize unbibium. In 2008, it was claimed to have been discovered in na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) proce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirac Sea
The Dirac sea is a theoretical model of the vacuum as an infinite sea of particles with negative energy. It was first postulated by the British physicist Paul Dirac in 1930 to explain the anomalous negative-energy quantum states predicted by the Dirac equation for relativistic electrons (electrons traveling near the speed of light). The positron, the antimatter counterpart of the electron, was originally conceived of as a hole in the Dirac sea, before its experimental discovery in 1932.This was not the original intent of Dirac though, as the title of his 1930 paper (''A Theory of Electrons and Protons'') indicates. But it soon afterwards became clear that the mass of holes must be that of the electron. In hole theory, the solutions with negative time evolution factors are reinterpreted as representing the positron, discovered by Carl Anderson. The interpretation of this result requires a Dirac sea, showing that the Dirac equation is not merely a combination of special relativi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review
''Physical Review'' is a peer-reviewed scientific journal established in 1893 by Edward Nichols. It publishes original research as well as scientific and literature reviews on all aspects of physics. It is published by the American Physical Society (APS). The journal is in its third series, and is split in several sub-journals each covering a particular field of physics. It has a sister journal, ''Physical Review Letters'', which publishes shorter articles of broader interest. History ''Physical Review'' commenced publication in July 1893, organized by Cornell University professor Edward Nichols and helped by the new president of Cornell, J. Gould Schurman. The journal was managed and edited at Cornell in upstate New York from 1893 to 1913 by Nichols, Ernest Merritt, and Frederick Bedell. The 33 volumes published during this time constitute ''Physical Review Series I''. The American Physical Society (APS), founded in 1899, took over its publication in 1913 and star ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirac Equation
In particle physics, the Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin- massive particles, called "Dirac particles", such as electrons and quarks for which parity is a symmetry. It is consistent with both the principles of quantum mechanics and the theory of special relativity, and was the first theory to account fully for special relativity in the context of quantum mechanics. It was validated by accounting for the fine structure of the hydrogen spectrum in a completely rigorous way. The equation also implied the existence of a new form of matter, ''antimatter'', previously unsuspected and unobserved and which was experimentally confirmed several years later. It also provided a ''theoretical'' justification for the introduction of several component wave functions in Pauli's phenomenological theory of spin. The wave functions in the Dirac theo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theory Of Relativity
The theory of relativity usually encompasses two interrelated theories by Albert Einstein: special relativity and general relativity, proposed and published in 1905 and 1915, respectively. Special relativity applies to all physical phenomena in the absence of gravity. General relativity explains the law of gravitation and its relation to the forces of nature. It applies to the cosmological and astrophysical realm, including astronomy. The theory transformed theoretical physics and astronomy during the 20th century, superseding a 200-year-old Classical mechanics, theory of mechanics created primarily by Isaac Newton. It introduced concepts including 4-dimensional spacetime as a unified entity of space and time in physics, time, relativity of simultaneity, kinematics, kinematic and gravity, gravitational time dilation, and length contraction. In the field of physics, relativity improved the science of elementary particles and their fundamental interactions, along with ushering in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Cloud
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital, p orb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Shell Model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model is a model of the atomic nucleus which uses the Pauli exclusion principle to describe the structure of the nucleus in terms of energy levels. The first shell model was proposed by Dmitri Ivanenko (together with E. Gapon) in 1932. The model was developed in 1949 following independent work by several physicists, most notably Eugene Paul Wigner, Maria Goeppert Mayer and J. Hans D. Jensen, who shared the 1963 Nobel Prize in Physics for their contributions. The nuclear shell model is partly analogous to the atomic shell model, which describes the arrangement of electrons in an atom in that filled shell results in better stability. When adding nucleons (protons or neutrons) to a nucleus, there are certain points where the binding energy of the next nucleon is significantly less than the last one. This observation that there are specific magic quantum numbers of nucleons (2, 8, 20, 28, 50, 82, 126) wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |