|
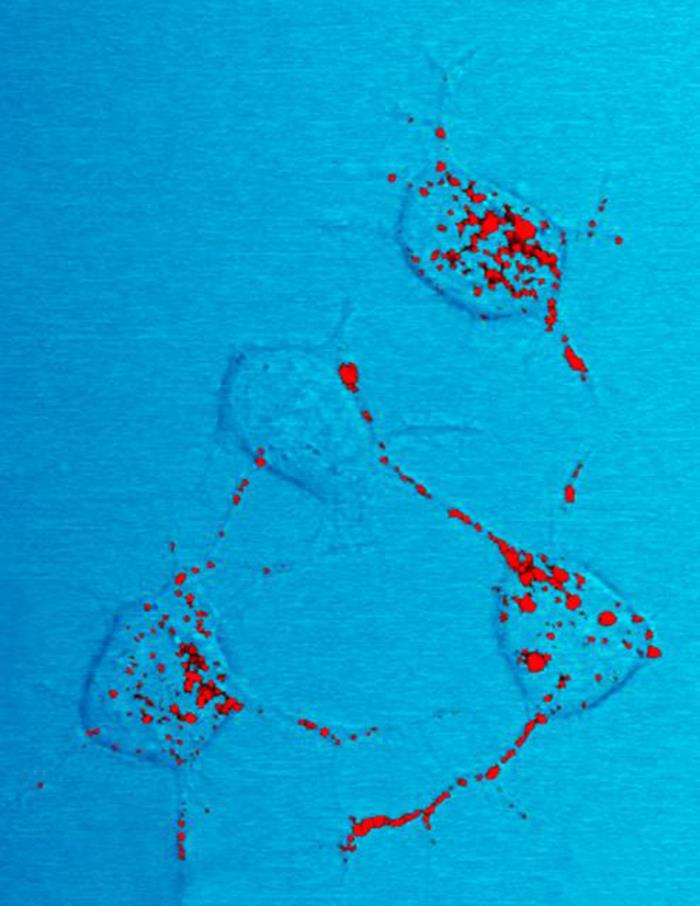
Sup35p
Sup35p is the ''Saccharomyces cerevisiae'' (a yeast) eukaryotic translation release factor. More specifically, it is the yeast eukaryotic release factor 3 (eRF3), which forms the translation termination complex with eRF1 (Sup45p in yeast). This complex recognizes and catalyzes the release of the nascent polypeptide chain when the ribosome encounters a stop codon. While eRF1 recognizes stop codons, eRF3 facilitates the release of the polypeptide chain through GTP hydrolysis. Partial loss of function results in nonsense suppression, in which stop codons are ignored and proteins are abnormally synthesized with carboxyl terminal extensions. Complete loss of function is fatal. History Sup35p was shown to propagate in a prion form in 1994 by Reed Wickner. For this reason it is an intensely studied protein. When yeast cells harbor Sup35p in the prion state the resulting phenotype is known as SI+ In SI+cells Sup35p exists in an amyloid state that can be propagated and passed to daught ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungal Prions
A fungal prion is a prion that infects fungal hosts. Fungal prions are naturally occurring proteins that can switch between multiple, structurally distinct conformations, at least one of which is self-propagating and transmissible to other prions. This transmission of protein state represents an epigenetic phenomenon where information is encoded in the protein structure itself, instead of in nucleic acids. Several prion-forming proteins have been identified in fungi, primarily in the yeast ''Saccharomyces cerevisiae''. These fungal prions are generally considered benign, and in some cases even confer a selectable advantage to the organism. Fungal prions have provided a model for the understanding of disease-forming mammalian prions. Study of fungal prions has led to a characterisation of the sequence features and mechanisms that enable prion domains to switch between functional and amyloid-forming states. Sequence features Prions are formed by portable, transmissible prion domain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evolutionary Capacitor
Evolutionary capacitance is the storage and release of variation, just as electric capacitors store and release charge. Living systems are robust to mutations. This means that living systems accumulate genetic variation without the variation having a phenotypic effect. But when the system is disturbed (perhaps by stress), robustness breaks down, and the variation has phenotypic effects and is subject to the full force of natural selection. An evolutionary capacitor is a molecular switch mechanism that can "toggle" genetic variation between hidden and revealed states. If some subset of newly revealed variation is adaptive, it becomes fixed by genetic assimilation. After that, the rest of variation, most of which is presumably deleterious, can be switched off, leaving the population with a newly evolved advantageous trait, but no long-term handicap. For evolutionary capacitance to increase evolvability in this way, the switching rate should not be faster than the timescale of genetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sup45p
Sup45p is the ''Saccharomyces cerevisiae'' (a yeast) eukaryotic translation termination factor. More specifically, it is the yeast eukaryotic release factor 1 (eRF1). Its job is to recognize stop codons in RNA and bind to them. It binds to the Sup35p protein and then takes on the shape of a tRNA Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ... molecule so that it can safety incorporate itself into the A site of the Ribosome to disruptits flow and "release" the protein and end translation. Notes Saccharomyces cerevisiae genes Protein biosynthesis {{Protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prions
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It is not known what causes a normal protein to misfold, but the resulting abnormal three-dimensional structure confers infectious properties by collapsing nearby protein molecules into the same shape. The word ''prion'' is derived from the term, "proteinaceous infectious particle". In comparison to all other known infectious agents such as viroids, viruses, bacteria, fungi, and parasites, all of which contain nucleic acids ( DNA, RNA, or both), the hypothesized role of a protein as an infectious agent stands in contrast. Prion isoforms of the prion protein (PrP), whose specific function is uncertain, are hypothesized as the cause of transmissible spongiform encephalopathies (TSEs), including scrapie in sheep, chronic wasting disease (C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prion
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It is not known what causes a normal protein to misfold, but the resulting abnormal three-dimensional structure confers infectious properties by collapsing nearby protein molecules into the same shape. The word ''prion'' is derived from the term, "proteinaceous infectious particle". In comparison to all other known infectious agents such as viroids, viruses, bacteria, fungi, and parasites, all of which contain nucleic acids ( DNA, RNA, or both), the hypothesized role of a protein as an infectious agent stands in contrast. Prion isoforms of the prion protein (PrP), whose specific function is uncertain, are hypothesized as the cause of transmissible spongiform encephalopathies (TSEs), including scrapie in sheep, chronic wasting disease (CWD) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prion
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It is not known what causes a normal protein to misfold, but the resulting abnormal three-dimensional structure confers infectious properties by collapsing nearby protein molecules into the same shape. The word ''prion'' is derived from the term, "proteinaceous infectious particle". In comparison to all other known infectious agents such as viroids, viruses, bacteria, fungi, and parasites, all of which contain nucleic acids ( DNA, RNA, or both), the hypothesized role of a protein as an infectious agent stands in contrast. Prion isoforms of the prion protein (PrP), whose specific function is uncertain, are hypothesized as the cause of transmissible spongiform encephalopathies (TSEs), including scrapie in sheep, chronic wasting disease (CWD) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reed Wickner
Reed B. Wickner (born c. 1942) is an American yeast geneticist. In 1994 he proposed that the 'PSI''+and RE3phenotypes in ''Saccharomyces cerevisiae'', a form of budding yeast, were caused by prion forms of native proteins - specifically, the Sup35p and Ure2p proteins, respectively. Wickner graduated from Cornell University with a B.A. degree in 1962. He then went to medical school at Georgetown University and received his M.D. degree in 1966. He is a member of the National Academy of Sciences (NAS), the American Academy of Arts and Sciences AAAS) and the American Academy of Microbiology, and has been a fellow of the American Association for the Advancement of Science. He is (as of 2012) Chief of the Laboratory of Biochemistry and Genetics at the National Institute of Diabetes & Digestive & Kidney Diseases, part of the National Institutes of Health. His research interests pertain to prions and amyloid Amyloids are aggregates of proteins characterised by a fibrillar morphol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid
Amyloids are aggregates of proteins characterised by a Fibril, fibrillar morphology of 7–13 Nanometer, nm in diameter, a beta sheet (β-sheet) Secondary structure of proteins, secondary structure (known as cross-β) and ability to be Staining, stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal Protein structure, structure and physiology, physiological functions (Protein misfolding, misfolding) and form fibrous deposits in amyloid plaques around cells which can disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are Genetic disorder, familial. Others are only Genetic disorder, fam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Release Factor
A release factor is a protein that allows for the termination of translation by recognizing the termination codon or stop codon in an mRNA sequence. They are named so because they release new peptides from the ribosome. Background During translation of mRNA, most codons are recognized by "charged" tRNA molecules, called aminoacyl-tRNAs because they are adhered to specific amino acids corresponding to each tRNA's anticodon. In the standard genetic code, there are three mRNA stop codons: UAG ("amber"), UAA ("ochre"), and UGA ("opal" or "umber"). Although these stop codons are triplets just like ordinary codons, they are not decoded by tRNAs. It was discovered by Mario Capecchi in 1967 that, instead, tRNAs do not ordinarily recognize stop codons at all, and that what he named "release factor" was not a tRNA molecule but a protein. Later, it was demonstrated that different release factors recognize different stop codons. Classification There are two classes of release factor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translation (genetics)
In molecular biology and genetics, translation is the process in which ribosomes in the cytoplasm or endoplasmic reticulum synthesize proteins after the process of transcription of DNA to RNA in the cell's nucleus. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later folds into an active protein and performs its functions in the cell. The ribosome facilitates decoding by inducing the binding of complementary tRNA anticodon sequences to mRNA codons. The tRNAs carry specific amino acids that are chained together into a polypeptide as the mRNA passes through and is "read" by the ribosome. Translation proceeds in three phases: # Initiation: The ribosome assembles around the target mRNA. The first tRNA is attached at the start codon. # Elongation: The last tRNA validated by the small ribosomal subunit (''accommodation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenine Pathway
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. Both adenine and guanine are derived from the nucleotide inosine monophosphate (IMP), which is the first compound in the pathway to have a completely formed purine ring system. IMP Inosine monophosphate is synthesized on a pre-existing ribose-phosphate through a complex pathway (as shown in the figure on the right). The source of the carbon and nitrogen atoms of the purine ring, 5 and 4 respectively, come from multiple sources. The amino acid glycine contributes all its carbon (2) and nitrogen (1) atoms, with additional nitrogen atoms from glutamine (2) and aspartic acid (1), and additional carbon atoms from formyl groups (2), which are transferred from the coenzyme tetrahydrofolate as 10-formyltetrahydrofolate, and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenine
Adenine () ( symbol A or Ade) is a nucleobase (a purine derivative). It is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The three others are guanine, cytosine and thymine. Its derivatives have a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate (ATP) and the cofactors nicotinamide adenine dinucleotide (NAD), flavin adenine dinucleotide (FAD) and Coenzyme A. It also has functions in protein synthesis and as a chemical component of DNA and RNA. The shape of adenine is complementary to either thymine in DNA or uracil in RNA. The adjacent image shows pure adenine, as an independent molecule. When connected into DNA, a covalent bond is formed between deoxyribose sugar and the bottom left nitrogen (thereby removing the existing hydrogen atom). The remaining structure is called an ''adenine residue'', as part of a larger molecule. Adenosine is ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |