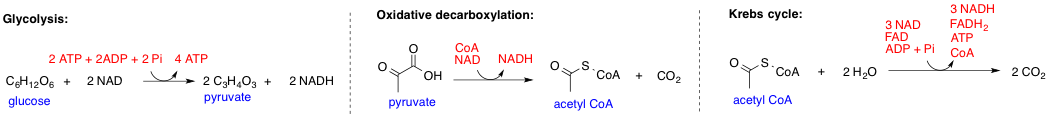|
Adenine Pathway
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. Both adenine and guanine are derived from the nucleotide inosine monophosphate (IMP), which is the first compound in the pathway to have a completely formed purine ring system. IMP Inosine monophosphate is synthesized on a pre-existing ribose-phosphate through a complex pathway (as shown in the figure on the right). The source of the carbon and nitrogen atoms of the purine ring, 5 and 4 respectively, come from multiple sources. The amino acid glycine contributes all its carbon (2) and nitrogen (1) atoms, with additional nitrogen atoms from glutamine (2) and aspartic acid (1), and additional carbon atoms from formyl groups (2), which are transferred from the coenzyme tetrahydrofolate as 10-formyltetrahydrofolate, and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell. These enzymes often require dietary minerals, vitamins, and other cofactors to function. Different metabolic pathways function based on the position within a eukaryotic cell and the significance of the pathway in the given compartment of the cell. For instance, the, electron transport chain, and oxidative phosphorylation all take place in the mitochondrial membrane. In contrast, glycolysis, pentose phosphate pathway, and fatty acid biosynthesis all occur in the cytosol of a cell. There are two types of metabolic pathways that are character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PRPP
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase: : It plays a role in transferring phospho-ribose groups in several reactions, some of which are salvage pathways: In '' de novo'' generation of purines, the enzyme amidophosphoribosyltransferase acts upon PRPP to create phosphoribosylamine. The histidine biosynthesis pathway involves the reaction between PRPP and ATP, which activates the latter to ring cleavage. Carbon atoms from ribose in PRPP form the linear chain and part of the imidazole ring in histidine. The same is true for the biosynthesis of tryptophan, with the fir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosylglycinamide Formyltransferase
Phosphoribosylglycinamide formyltransferase (, ''2-amino-N-ribosylacetamide 5'-phosphate transformylase'', ''GAR formyltransferase'', ''GAR transformylase'', ''glycinamide ribonucleotide transformylase'', ''GAR TFase'', ''5,10-methenyltetrahydrofolate:2-amino-N-ribosylacetamide ribonucleotide transformylase'') is an enzyme with systematic name ''10-formyltetrahydrofolate:5'-phosphoribosylglycinamide N-formyltransferase''. This enzyme catalyses the following chemical reaction : 10-formyltetrahydrofolate + N1-(5-phospho-D-ribosyl)glycinamide \rightleftharpoons tetrahydrofolate + N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide This THF dependent enzyme catalyzes a nucleophilic acyl substitution of the formyl group from 10-formyltetrahydrofolate (fTHF) to N1-(5-phospho-D-ribosyl)glycinamide (GAR) to form N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide (fGAR) as shown above. This reaction plays an important role in the formation of purine through the ''de novo'' purine biosynthesis pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosylamine—glycine Ligase
''Phosphoribosylamine—glycine ligase'', also known as glycinamide ribonucleotide synthetase (GARS), () is an enzyme that catalyzes the chemical reaction :ATP + 5-phospho-D-ribosylamine + glycine \rightleftharpoons ADP + phosphate + which is the second step in purine biosynthesis. The 3 substrates of this enzyme are ATP, 5-phospho-D-ribosylamine, and glycine, whereas its 3 products are ADP, phosphate, and . This enzyme belongs to the family of ligases, specifically those forming generic carbon-nitrogen bonds. In bacteria, GARS is a monofunctional enzyme (encoded by the purD gene). The purD genes often contain PurD RNA motif in their 5' UTR. In yeast, GARS is part of a bifunctional enzyme (encoded by the ADE5/7 gene) in conjunction with phosphoribosylformylglycinamidine cyclo-ligase (AIRS). In higher eukaryotes, including humans, GARS is part of a trifunctional enzyme in conjunction with AIRS and with phosphoribosylglycinamide formyltransferase (GART), forming GARS- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycineamide Ribonucleotide
Glycineamide ribonucleotide (or GAR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from GAR. : GAR is the product of the enzyme phosphoribosylamine—glycine ligase acting on phosphoribosylamine (PRA) to combine it with glycine in a process driven by ATP. The reaction, forms an amide bond: : + + ATP → + ADP + Pi The biosynthesis pathway next adds a formyl group from 10-formyltetrahydrofolate to GAR, catalysed by phosphoribosylglycinamide formyltransferase in reaction and producing formylglycinamide ribotide (FGAR): :GAR + 10-formyltetrahydrofolate → FGAR + tetrahydrofolate See also * 5-Aminoimidazole ribotide * Purine metabolism Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosylamine
Phosphoribosylamine (PRA) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from PRA. : It is the product of the enzyme amidophosphoribosyltransferase which attaches ammonia from glutamine to phosphoribosyl pyrophosphate (PRPP) at its anomeric carbon: : + → + + PPi The biosynthesis pathway next combines PRA with glycine in a process driven by ATP giving glycineamide ribonucleotide (GAR). The enzyme phosphoribosylamine—glycine ligase catalyses the reaction forming an amide bond: : + + ATP → + ADP + Pi See also * 5-Aminoimidazole ribotide * Purine metabolism Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to r ... ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Monophosphate
Guanosine monophosphate (GMP), also known as 5′-guanidylic acid or guanylic acid (conjugate base guanylate), is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase guanine; hence it is a ribonucleoside monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation. As an acyl substituent, it takes the form of the prefix guanylyl-. ''De novo'' synthesis GMP synthesis starts with D-ribose 5′-phosphate, a product of the pentose phosphate pathway. The synthesis proceeds by the gradual formation of the purine ring on carbon-1 of ribose, with CO2, glutamine, glycine, aspartate and one-carbon derivatives of tetrahydrofolate donating various elements towards the building of the ring As inhibitor of guanosine monophosphate synthesis in experimental models, the glutamine analogue DON can be used.''Ahluwalia GS et alMet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Monophosphate
Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine; it is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-. AMP plays an important role in many cellular metabolic processes, being interconverted to Adenosine diphosphate, ADP and/or Adenosine triphosphate, ATP. AMP is also a component in the synthesis of RNA. AMP is present in all known forms of life. Production and degradation AMP does not have the high energy phosphoanhydride bond associated with ADP and ATP. AMP can be produced from Adenosine diphosphate, ADP: : 2 ADP → ATP + AMP Or AMP may be produced by the hydrolysis of one high energy phosphate bond of ADP: : ADP + H2O → AMP + phosphate, Pi AMP can also be formed by hydrolysis of Adenosine triphosphate, ATP into AMP and pyrophosphate: : ATP + H2O → AMP + pyrophosphate, PPi When RNA i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidophosphoribosyltransferase
Amidophosphoribosyltransferase (ATase), also known as glutamine phosphoribosylpyrophosphate amidotransferase (GPAT), is an enzyme responsible for catalyzing the conversion of 5-phosphoribosyl-1-pyrophosphate (PRPP) into 5-phosphoribosyl-1-amine (PRA), using the amine group from a glutamine side-chain. This is the committing step in de novo purine synthesis. In humans it is encoded by the ''PPAT'' (phosphoribosyl pyrophosphate amidotransferase) gene. ATase is a member of the purine/pyrimidine phosphoribosyltransferase family. Structure and function The enzyme consists of two domains: a glutaminase domain that produces ammonia from glutamine by hydrolysis and a phosphoribosyltransferase domain that binds the ammonia to ribose-5-phosphate. Coordination between the two active sites of enzyme give it special complexity. The glutaminase domain is homologous to other N-terminal nucleophile (Ntn) hydrolases such as carbamoyl phosphate synthetase (CPSase). Nine invariant residues am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrophosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |