|
Strictosidine Synthase
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction: :3-α(''S'')-strictosidine + H2O = tryptamine + secologanin Since the condensation of tryptamine and secologanin is the first committed step in alkaloid synthesis, strictosidine synthase plays a fundamental role for the great majority of the indole-alkaloid pathways. This enzyme belongs to the family of lyases, specifically amine lyases, which cleave carbon-nitrogen bonds. It can be isolated from several alkaloid-producing plants from the family Apocynaceae (e.g. ''Catharanthus roseus'', ''Voacanga africana''). The systematic name of this enzyme class is 3-α(''S'')-strictosidine tryptamine-lyase (secologanin-forming). Other names in common use include strictosidine synthetase, STR, and 3-α(''S'')-strictosidine tryptamine-lyase. Originally isolated from the plant ''Rauvolfia serpen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a class of basic BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ..., natural product, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms includi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strictosidine Synthase Mechanism (2-28-13, V3)
Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids. Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine. Biosynthetic pathways help to define the subgroups of strictosidine derivatives. Distribution Strictosidine is found in the following plant families: * Apocynaceae Here especially in Rhazya stricta ''Rhazya stricta'' (Persian: اشورک Eshvarak) is a native poisonous plant in Southern Iran, Afghanistan, Pakistan, India, Iraq, Oman, Yemen, and Saudi Arabia.Muẓaffariān, Walī Allāh. 1996. A dictionary of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytochemistry (journal)
''Phytochemistry'' is a peer-reviewed scientific journal covering pure and applied plant chemistry, plant biochemistry and molecular biology. It is published by Elsevier and is an official publication for the Phytochemical Society of Europe, the Phytochemical Society of North America, and the Phytochemical Society of Asia. A sister journal ''Phytochemistry Letters'' is published since 2008. Abstracting and indexing ''Phytochemistry'' is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 4.072. References External links {{Official website, http://www.journals.elsevier.com/phytochemistry/ Biochemistry journals Botany journals Elsevier academic journals En ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinblastine
Vinblastine (VBL), sold under the brand name Velban among others, is a chemotherapy medication, typically used with other medications, to treat a number of types of cancer. This includes Hodgkin's lymphoma, non-small cell lung cancer, bladder cancer, brain cancer, melanoma, and testicular cancer. It is given by injection into a vein. Most people experience some side effects. Commonly it causes a change in sensation, constipation, weakness, loss of appetite, and headaches. Severe side effects include low blood cell counts and shortness of breath. It should not be given to people who have a current bacterial infection. Use during pregnancy will likely harm the baby. Vinblastine works by blocking cell division. Vinblastine was isolated in 1958. An example of a natural herbal remedy that has since been developed into a conventional medicine, vinblastine was originally obtained from the Madagascar periwinkle. It is on the World Health Organization's List of Essential Medicin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vincristine
Vincristine, also known as leurocristine and marketed under the brand name Oncovin among others, is a chemotherapy medication used to treat a number of types of cancer. This includes acute lymphocytic leukemia, acute myeloid leukemia, Hodgkin's disease, neuroblastoma, and small cell lung cancer among others. It is given intravenously. Most people experience some side effects from vincristine treatment. Commonly it causes a change in sensation, hair loss, constipation, difficulty walking, and headaches. Serious side effects may include neuropathic pain, lung damage, or low white blood cells which increases the risk of infection. Use during pregnancy may result in birth defects. It works by stopping cells from dividing properly. It is vital that it not be given intrathecally, as this causes paralysis and in most cases, death. Vincristine was first isolated in 1961. It is on the World Health Organization's List of Essential Medicines. It is a vinca alkaloid that can be obta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camptothecin
Camptothecin (CPT) is a topoisomerase inhibitor. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of '' Camptotheca acuminata'' (Camptotheca, Happy tree), a tree native to China used in traditional Chinese medicine. It has been used clinically more recently in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical, with good results. Four CPT analogues have been approved and are used in cancer chemotherapy today, topotecan, irinotecan, belotecan, and trastuzumab deruxtecan. Camptothecin ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to '' Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects. It can be taken by mouth or intravenously. Malaria resistance to quinine occurs in certain areas of the world. Quinine is also used as an ingredient in tonic water to impart a bitter taste. Common side effects include headache, ringing in the ears, vision issues, and sweating. More severe side effects include deafness, low blood platelets, and an irregular heartbeat. Use can make one more prone to sunburn. While it is unclear if use during pregnancy causes harm to the baby, treating malaria during pregnancy with quinine when appropriate is still recommended. Quinine is an alkaloid, a naturally occurring chemical compound. How it works as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jasmonate
Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers. History The isolation of methyl jasmonate (MeJa) from jasmine oil derived from '' Jasminum grandiflorum'' led to the discovery of the molecular structure of jasmonates and their name in 1962 while jasmonic acid itself was isolated from '' Lasiodiplodia theobromae'' by Alderidge et al in 1971. Biosynthesis Biosynthesis is reviewed by Acosta and Farmer 2010, Wasternack and Hause 2013, and Wasternack and Song 2017. Jasmonates (JA) are oxylipins, i.e. derivatives of oxygenated fatty acid. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
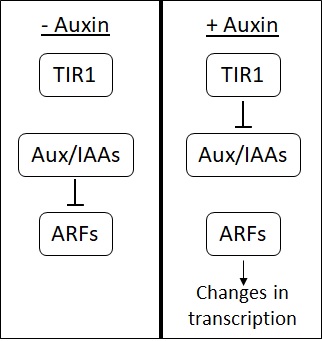
Auxin
Auxins (plural of auxin ) are a class of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their role in plant growth in the 1920s. Kenneth V. Thimann became the first to isolate one of these phytohormones and to determine its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, ''Phytohormones'', in 1937. Overview Auxins were the first of the major plant hormones to be discovered. They derive their name from the Greek word αυξειν (''auxein'' – "to grow/increase"). Auxin is present in all parts of a plant, although in very different concentrations. The concentration in each position is crucial developmental information, so it is subject to tight regulation through both ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, cryo-electron microscopy, and submitted by biologists and biochemists from around the world, are freely accessible on the Internet via the websites of its member organisations (PDBe, PDBj, RCSB, and BMRB). The PDB is overseen by an organization called the Worldwide Protein Data Bank, wwPDB. The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other databases use protein structures deposited in the PDB. For example, SCOP and CATH classify protein structures, while PDBsum provides a graphic overview of PDB entries using information from other sources, such as Gene ontology. History Two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of poly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)