|
Stemmadenine
Stemmadenine is a terpene indole alkaloid. Stemmadenine is believed to be formed from preakuammicine by a carbon-carbon bond cleavage. Cleavage of a second carbon-carbon bond is thought to form dehydrosecodine. The enzymes forming stemmadenine and using it as a substrate remain unknown to date. It is thought to be intermediate compound in many different biosynthetic pathways such as in Aspidosperma species. Many alkaloids are proposed to be produced through intermediate stemmadenine. Some of them are: * Catharanthine and Tabersonine in ''Catharanthus roseus'' * Subincanadines D-F in '' Aspidosperma subincanum'' It is also present as product in plant like in '' Tabernaemontana dichotoma'' seeds. Pharmacology It has hypotensive and weak muscle relaxant properties. See also *Secologanin *Strictosidine Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catharanthine
Catharanthine is a terpene indole alkaloid produced by the medicinal plant ''Catharanthus roseus'' and '' Tabernaemontana divaricata''. Catharanthine is derived from strictosidine, but the exact mechanism by which this happens is currently unknown. Catharanthine is one of the two precursors that form vinblastine, the other being vindoline. Pharmacology (+)-Catharanthine competitively inhibits α9α10 nAChRs with potencies higher than that at α3β4 and α4β2 nAChRs and directly blocks CaV2.2. Catharanthine alkaloids are non competitive antagonist of muscle type nAChRs and this is thought to be the case due to presence of catharanthine moiety in those compounds. In ''in vitro'' study, it increased the levels of cAMP by inhibiting cAMP phosphodiesterase in brain. It is a potent inhibitor of TRPM8, similar to BCTC. Structural analysis of catharanthine shows activity on TRPM8, TRPA1, and butyrylcholinesterase. See also * Akuammicine * Conopharyngine * Stemmadenine St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tabersonine
Tabersonine is a terpene indole alkaloid found in the medicinal plant ''Catharanthus roseus'' and also in the genus Voacanga (both taxa belonging to the alkaloid-rich family Apocynaceae). Tabersonine is hydroxylated at the 16 position by the enzyme tabersonine 16-hydroxylase (T16H) to form 16-hydroxytabersonine.St-Pierre and De Luca (1995) A Cytochrome P-450 Monooxygenase Catalyzes the First Step in the Conversion of Tabersonine to Vindoline in Catharanthus roseus. Plant Physiology. 109(1). 131-139 The enzyme leading to its formation is currently unknown. Tabersonine is the first intermediate leading to the formation of vindoline one of the two precursors required for vinblastine biosynthesis. See also *Conopharyngine * Tabernanthine *Vinblastine Vinblastine (VBL), sold under the brand name Velban among others, is a chemotherapy medication, typically used with other medications, to treat a number of types of cancer. This includes Hodgkin's lymphoma, non-small cell lung c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidine Alkaloids
Piperidine alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from piperidine. Alkaloids with a piperidine building block are widespread and are usually further subdivided according to their occurrence and biogenetic origin. The most important representative of piperidine alkaloids is piperine, which is responsible for the pungent taste of pepper. The piperidine alkaloids also include the sedum alkaloids (e.g. sedamine), pelletierine, the lobelia alkaloids (e.g. lobeline), the conium alkaloids (such as coniine) and the pinus alkaloids. Piperin.svg, Piperine Lobeline Structural Formula V2.svg, Lobeline (S)-Coniine Structural Formula V.1.svg, (''S'')-Coniine (2R,8R)-Sedamine Structural Formula V4.svg, Sedamin Solenopsin Structural Formula V.1.svg, Solenopsin Solenopsin is a lipophilic alkaloid with the molecular formula C17H35N found in the venom of fire ants (''Solenopsis''). It is considered the primary toxin in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
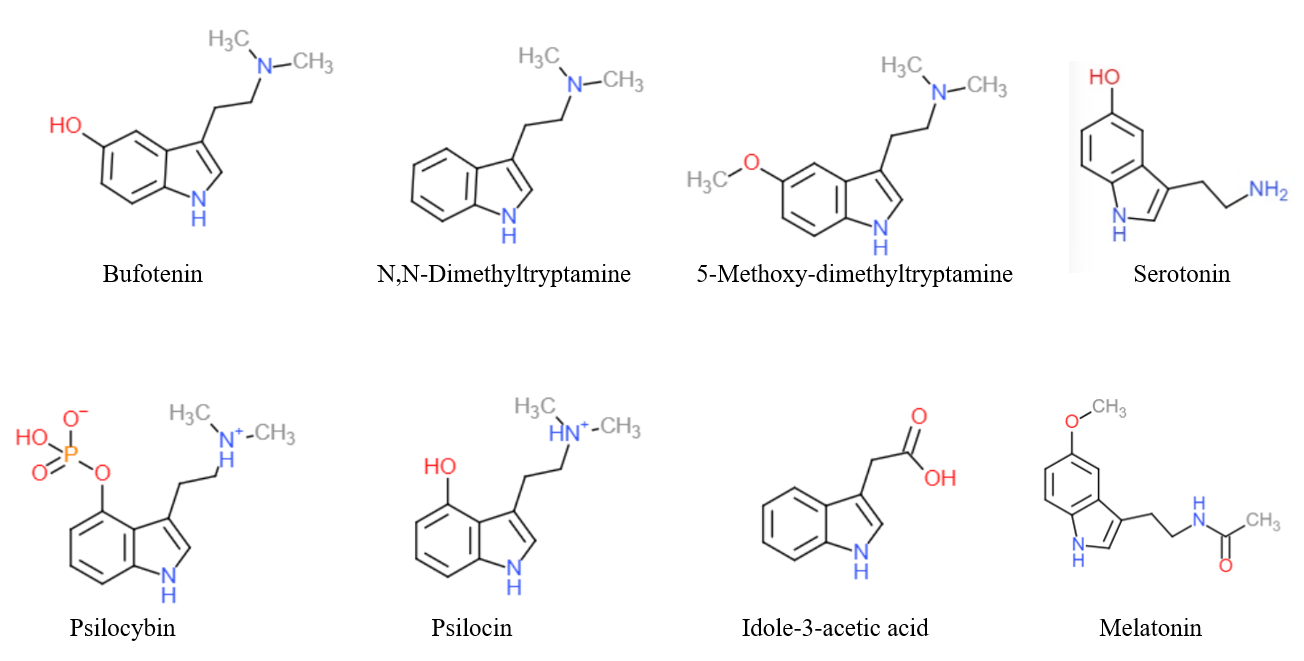
Tryptamine Alkaloids
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strictosidine
Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids. Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine. Biosynthetic pathways help to define the subgroups of strictosidine derivatives. Distribution Strictosidine is found in the following plant families: *Apocynaceae Here especially in Rhazya stricta and Catharanthus roseus. *Loganiaceae *Rubiaceae *Icacinaceae *Nyssaceae *Alangiaceae Recent efforts in metabolic engineering have permitted the synthesis of strictosidine by yeast (''Saccharomyces cerevisiae ''Saccharomyces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secologanin
Secologanin is a secoiridoid monoterpene synthesized from geranyl pyrophosphate in the mevalonate pathway. Secologanin then proceeds with dopamine or tryptamine to form ipecac and terpene indole alkaloids, respectively. Biosynthesis Secologanin biosynthesis begins from geranyl pyrophosphate (GPP) taken from the mevalonate pathway used to make terpenoids. Recent efforts have characterized the entire secologanin biosynthetic pathway. Secologanin is formed from loganin through the action of the enzyme secologanin synthase In enzymology, a secologanin synthase (, was wrongly classified as in the past) is an enzyme that catalyzes the chemical reaction :loganin + NADPH + H+ + O2 \rightleftharpoons secologanin + NADP+ + 2 H2O The 4 substrates of this enzyme are lo .... Secologanin is then able to proceed onto produce ipecac and terpene indole alkaloids. References {{Reflist Glucosides Methyl esters Aldehydes Vinyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tabernaemontana Dichotoma
''Tabernaemontana dichotoma'', commonly known as Eve's apple, is a plant in the dogbane family Apocynaceae. The specific epithet refers to the species' dichotomous A dichotomy is a partition of a whole (or a set) into two parts (subsets). In other words, this couple of parts must be * jointly exhaustive: everything must belong to one part or the other, and * mutually exclusive: nothing can belong simultan ... branches. Description ''Tabernaemontana dichotoma'' grows as a shrub or tree, measuring from tall, rarely to . The trunk measures up in diameter. The plant's latex, fruit and seeds are all poisonous. Distribution and habitat ''Tabernaemontana dichotoma'' is native to Sri Lanka. It occurs at altitudes to around . References dichotoma Flora of Sri Lanka Plants described in 1829 Poisonous plants {{Apocynaceae-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspidosperma Subincanum
''Aspidosperma subincanum'' is a timber tree native to Brazil and Bolivia. It is common in Cerrado vegetation in Brazil. It was first described by Carl Friedrich Philipp von Martius Carl Friedrich Philipp (Karl Friedrich Philipp) von Martius (17 April 1794 – 13 December 1868) was a German botanist and explorer. Life Martius was born at Erlangen, the son of Prof Ernst Wilhelm Martius, court apothecary. He graduated PhD f ... in 1838.Morokawa, R. & al. (2013). Apocynaceae s. str. do Parque Nacional da Serra da Canastra, Minas Gerais, Brasil. Rodriguésia; Revista do Instituto de Biologia Vegetal, Jardim Botânico e Estaçao Biologica do Itatiaya 64: 179-199. References subincanum Trees of South America Plants described in 1838 {{Tree-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catharanthus Roseus
''Catharanthus roseus'', commonly known as bright eyes, Cape periwinkle, graveyard plant, Madagascar periwinkle, old maid, pink periwinkle, rose periwinkle, is a species of flowering plant in the family Apocynaceae. It is native and endemic to Madagascar, but grown elsewhere as an ornamental and medicinal plant. It is a source of the drugs vincristine and vinblastine, used to treat cancer. It was formerly included in the genus ''Vinca'' as ''Vinca rosea''. It has many vernacular names among which are ''arivotaombelona'' or ''rivotambelona'', ''tonga'', ''tongatse'' or ''trongatse'', ''tsimatiririnina'', and ''vonenina''. Synonyms Two varieties are recognized * ''Catharanthus roseus'' var. ''roseus'' : Synonymy for this variety ::''Catharanthus roseus'' var. ''angustus'' Steenis ex Bakhuizen f. :: ''Catharanthus roseus'' var. ''albus'' G.DonG.Don, Gen. Hist. 4(1): 95. 1837. :: ''Catharanthus roseus'' var. ''occellatus'' G.Don :: ''Catharanthus roseus'' var. ''nanus'' Markgr. :: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Ethnopharmacology
The ''Journal of Ethnopharmacology'' is a peer-reviewed medical journal covering the traditional medicinal use of plants and other substances. It is the official journal of the International Society for Ethnopharmacology. The journal is included in the Index Medicus (MEDLINE MEDLINE (Medical Literature Analysis and Retrieval System Online, or MEDLARS Online) is a bibliographic database of life sciences and biomedical information. It includes bibliographic information for articles from academic journals covering medic ...). References External links * International Society for Ethnopharmacology Pharmacology journals Elsevier academic journals Publications established in 1979 English-language journals {{ethno-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |