|
Staphylococcal Enterotoxin
An enterotoxin is a protein exotoxin released by a microorganism that targets the intestines. Enterotoxins are chromosomally encoded or plasmid encoded exotoxins that are produced and secreted from several bacterial organisms. They are heat labile (>60⁰), and are of low molecular weight and water-soluble. Enterotoxins are frequently cytotoxic and kill cells by altering the apical membrane permeability of the mucosal (epithelial) cells of the intestinal wall. They are mostly pore-forming toxins (mostly chloride pores), secreted by bacteria, that assemble to form pores in cell membranes. This causes the cells to die. Clinical significance Enterotoxins have a particularly marked effect upon the gastrointestinal tract, causing traveler's diarrhea and food poisoning. The action of enterotoxins leads to increased chloride ion permeability of the apical membrane of intestinal mucosal cells. These membrane pores are activated either by increased cAMP or by increased calcium ion con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exotoxins
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface. They may exert their effect locally or produce systemic effects. Well-known exotoxins include: botulinum toxin produced by ''Clostridium botulinum''; ''Corynebacterium diphtheriae'' toxin, produced during life-threatening symptoms of diphtheria; tetanospasmin produced by ''Clostridium tetani''. The toxic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding (molecular)
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding. In some cases, the associations can be quite strong—for example, the protein streptavidin and the vitamin biotin have a dissociation constant (reflecting the ratio between bound and free biotin) on the order of 10−14—and so the reactions are effectively irreversible. The result of molecular binding is sometimes the formation of a molecular complex in which the attractive forces holding the components together are generally non-covalent, and thus are normally energetically weaker than covalent bonds. Molecular binding occurs in biological complexes (e.g., between pairs or sets of proteins, or between a protein and a small molecule ligand it binds) and also in abiologic chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staphylococcal Enterotoxin B
In the field of molecular biology, enterotoxin type B, also known as Staphylococcal enterotoxin B (SEB), is an enterotoxin produced by the gram-positive bacteria ''Staphylococcus aureus''. It is a common cause of food poisoning, with severe diarrhea, nausea and intestinal cramping often starting within a few hours of ingestion. Being quite stable, the toxin may remain active even after the contaminating bacteria are killed. It can withstand boiling at 100 °C for a few minutes. Gastroenteritis occurs because SEB is a superantigen, causing the immune system to release a large amount of cytokines that lead to significant inflammation. Additionally, this protein is one of the causative agents of toxic shock syndrome. Function The function of this protein is to facilitate the infection of the host organism. It is a virulence factor designed to induce pathogenesis. One of the major virulence exotoxins is the toxic shock syndrome toxin (TSST), which is secreted by the organis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a common three-dimensional structure that appears in a variety of different, evolutionarily unrelated molecules. A structural motif does not have to be associated with a sequence motif; it can be represented by different and completely unrelated sequences in different proteins or RNA. In nucleic acids Depending upon the sequence and other conditions, nucleic acids can form a variety of structural motifs which is thought to have biological significance. ;Stem-loop: Stem-loop intramolecular base pairing is a pattern that can occur in single-stranded DNA or, more commonly, in RNA. The structure is also known as a hairpin or hairpin loop. It occurs when two regions of the same strand, usually complementary in nucleotide sequence when read in opposite directions, base-pair to form a double helix that ends in an unpaired loop. The resulting structure is a key building block of many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Domain (biology)
In biological taxonomy, a domain ( or ) (Latin: ''regio''), also dominion, superkingdom, realm, or empire, is the highest taxonomic rank of all organisms taken together. It was introduced in the three-domain system of taxonomy devised by Carl Woese, Otto Kandler and Mark Wheelis in 1990. According to the domain system, the tree of life consists of either three domains such as Archaea, Bacteria, and Eukarya, or two domains consisting of Archaea and Bacteria, with Eukarya included in Archaea. The first two are all prokaryotes, single-celled microorganisms without a membrane-bound nucleus. All organisms that have a cell nucleus and other membrane-bound organelles are included in Eukarya. Non-cellular life is not included in this system. Alternatives to the three-domain system include the earlier two-empire system (with the empires Prokaryota and Eukaryota), and the eocyte hypothesis (with two domains of Bacteria and Archaea, with Eukarya included as a branch of Archaea). Term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-barrel
In protein structures, a beta barrel is a beta sheet composed of tandem repeats that twists and coils to form a closed toroidal structure in which the first strand is bonded to the last strand (hydrogen bond). Beta-strands in many beta-barrels are arranged in an antiparallel fashion. Beta barrel structures are named for resemblance to the barrels used to contain liquids. Most of them are water-soluble proteins and frequently bind hydrophobic ligands in the barrel center, as in lipocalins. Others span cell membranes and are commonly found in porins. Porin-like barrel structures are encoded by as many as 2–3% of the genes in Gram-negative bacteria. It has been shown that more than 600 proteins with various function (e.g., oxidase, dismutase, amylase) contain the beta barrel structure. In many cases, the strands contain alternating polar and non-polar (hydrophilic and hydrophobic) amino acids, so that the hydrophobic residues are oriented into the interior of the barrel to form a hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astbur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a polypeptide folds into its characteristic three-dimensional structure from a random coil. Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of mRNA to a linear chain of amino acids. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure. Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence (biology)
A sequence in biology is the one-dimensional ordering of monomers, covalently linked within a biopolymer; it is also referred to as the primary structure of a biological macromolecule. While it can refer to many different molecules, the term sequence is most often used to refer to a DNA sequence. See also * Protein sequence * DNA sequence * Genotype * Self-incompatibility in plants * List of geneticists * Human Genome Project * Dot plot (bioinformatics) * Multiplex Ligation-dependent Probe Amplification * Sequence analysis In bioinformatics, sequence analysis is the process of subjecting a DNA, RNA or peptide sequence to any of a wide range of analytical methods to understand its features, function, structure, or evolution. Methodologies used include sequence alig ... Molecular biology {{molecular-biology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
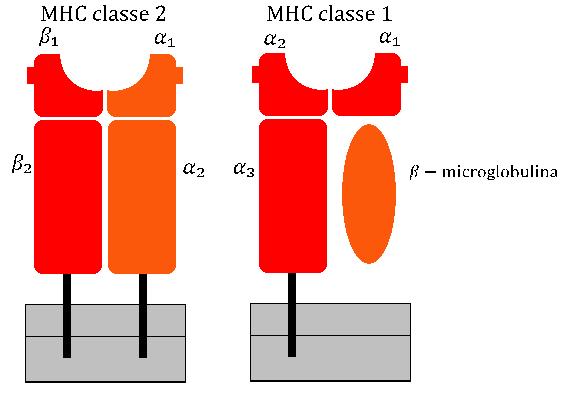
Major Histocompatibility Complex
The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules. This locus got its name because it was discovered via the study of transplanted tissue compatibility. Later studies revealed that tissue rejection due to incompatibility is only a facet of the full function of MHC molecules: binding an antigen derived from self-proteins, or from pathogens, and bringing the antigen presentation to the cell surface for recognition by the appropriate T-cells. MHC molecules mediate the interactions of leukocytes, also called white blood cells (WBCs), with other leukocytes or with body cells. The MHC determines donor compatibility for organ transplant, as well as one's susceptibility to autoimmune diseases. In a cell, protein molecules of the host's own phenotype or of other biologic entities ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |