|
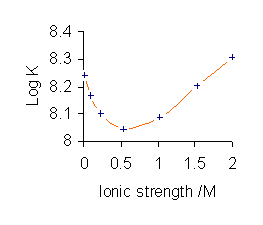
Stability Constants Of Complexes
In coordination chemistry, a stability constant (also called formation constant or binding constant) is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the Complex (chemistry), complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine. History Jannik Bjerrum (son of Niels Bjerrum) developed the first general method for the determination of stability constants of Metal ammine complex, metal-ammine complexes in 1941. The reasons why this occurred at such a late date, nearly 50 years after Alfred Werner had proposed the correct structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self-ionization Of Water
The self-ionization of water (also autoionization of water, autoprotolysis of water, autodissociation of water, or simply dissociation of water) is an ionization reaction in properties of water, pure water or in an aqueous solution, in which a water molecule, H2O, deprotonation, deprotonates (loses the nucleus of one of its hydrogen atoms) to become a hydroxide ion, OH−. The hydron (chemistry), hydrogen nucleus, H+, immediately protonation, protonates another water molecule to form a hydronium cation, H3O+. It is an example of autoprotolysis, and exemplifies the amphoterism, amphoteric nature of water. History and notation The self-ionization of water was first proposed in 1884 by Svante Arrhenius as part of the theory of ionic dissociation which he proposed to explain the Conductivity (electrolytic), conductivity of electrolytes including water. Arrhenius wrote the self-ionization as H2O H+ + OH-. At that time, nothing was yet known of atomic structure or subatomic particles, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in '' state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry. Indirect calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The dynamic energy budget theory explains why this procedure is corre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By definition, the Celsius scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15. The 19th century British scientist Lord Kelvin first developed and proposed the scale. It was often called the "absolute Celsius" scale in the early 20th century. The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the triple point of water. The Celsius, Fahrenheit, and Rankine scales were redefined in terms of the Kelvin scale using this definition. The 2019 revision of the SI now defines the kelvin in terms of energy by setting the Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of substance. The Boltzmann constant a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–volume work, pressure–volume work, that may be performed by a closed system, thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy is expressed as G(p,T) = U + pV - TS = H - TS where: * U is the internal energy of the system * H is the enthalpy of the system * S is the entropy of the system * T is the temperature of the system * V is the volume of the system * p is the pressure of the system (which must be equal to that of the surroundings for mechanical equilibrium). The Gibbs free energy change (, measured in joules in International System of Units, SI) is the ''maximum'' amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change and information systems including the transmission of information in telecommunication. Entropy is central to the second law of thermodynamics, which states that the entropy of an isolated system left to spontaneous evolution cannot decrease with time. As a result, isolated systems evolve toward thermodynamic equilibrium, where the entropy is highest. A consequence of the second law of thermodynamics is that certain processes are irreversible. The thermodynami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work (physics), work W that was done against constant external pressure P_\text to establish the system's physical dimensions from V_\text=0 to some final volume V_\text (as W=P_\text\Delta V), i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; Bond energy, bond, Lattice energy, lattice, solvation, and other chemical "energies" are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula , also written as or (Ph = ). It is one of the more common phosphine oxides. This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds. Structure and properties is structurally related to . As established by X-ray crystallography, the geometry around P is tetrahedral, and the P-O distance is 1.48 Å. Other modifications of have been found: For example, a monoclinic form crystalizes in the space group ''P''21/''c'' with Z = 4 and a = 15.066(1) Å, b = 9.037(2) Å, c = 11.296(3) Å, and β = 98.47(1)°.The orthorhombic modification crystallizes in the space group ''Pbca'' with Z = 4 and 29.089(3) Å, b = 9.1347(9), c = 11.261(1) Å. The oxygen center is relatively basic. The rigidity of the backbone and the basicity of the oxygen ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water (at a boiling point of ) and other chlorocarbons. History In 1794, physician Jan Rudolph Deiman, merchant Adriaan Paets van Troostwijk, chemist Anthoni Lauwerenburg, and botanist Nicolaas Bondt, under the name of Society of Dutch Chemists (), were the first to produce 1,2-dichloroethane from olefiant gas (oil-making gas, ethylene) and chlorine gas. Although the ''Gezelschap'' in practice did not do much in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers. Preparation and structure Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride: :SbCl3 + Cl2 → SbCl5 Gaseous SbCl5 has a trigonal bipyramidal structure. Reactions This compounds reacts with water to form antimony pentoxide and hydrochloric acid: :2 SbCl5 + 5 H2O → Sb2O5 + 10 HCl The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O. This compound forms adducts with many Lewis bases. SbCl5 is a soft Lewis acid and its ECW model parameters are EA = 3.64 and CA = 10.42. It is used as the standard Lewis acid in the Gutmann scale of Lewis basicity. It is also a strong oxidizing agent. For example aromatic ethers are oxidize ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Donor Number
In chemistry a donor number (DN) is a quantitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 (antimony pentachloride), in dilute solution in the noncoordinating solvent 1,2-dichloroethane with a zero DN. The units are kilocalories per mole for historical reasons. The donor number is a measure of the ability of a solvent to solvate cations and Lewis acids. The method was developed by V. Gutmann in 1976. Likewise Lewis acids are characterized by acceptor numbers (AN, see Gutmann–Beckett method). Typical solvent values are: * acetonitrile 14.1 kcal/mol (59.0 kJ/mol) * acetone 17 kcal/mol (71 kJ/mol) * methanol 19 kcal/mol (79 kJ/mol) * tetrahydrofuran 20 kcal/mol (84 kJ/mol) * dimethylformamide (DMF) 26.6 kcal/mol (111 kJ/mol) * dimethyl sulfoxide (DMSO) 29.8 kcal/mol (125 kJ/mol) * ethanol 31.5 kcal/mol (132 kJ/mol) * pyridine 33.1 kcal/mol (138 kJ/mol) * t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |