|
Sporolides
Sporolides A and B are polycyclic macrolides extracted from the obligate marine bacterium '' Salinispora tropica'', which is found in ocean sediment. They are composed of a chlorinated cyclopenta ndene ring and a cyclohexenone moiety. They were the second group of compounds (after salinosporamide A) isolated from '' Salinispora'', and were said to indicate the potential of marine actinomycetes as a source of novel secondary metabolites. The structures and absolute stereochemistries of both metabolites were elucidated using a combination of NMR spectroscopy and X-ray crystallography. The complex aromatic structure of the sporolides was hypothesized to be derived from an unstable nine-membered ring enediyne precursor, which could undergo Bergman cyclization to generate a para-benzyne intermediate. Nucleophilic attack by chloride would account for the 1:1 mixture of sporolide A and B and for the single chlorine in these enediyne-derived natural products. This proposed mechanism was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salinispora Tropica
''Salinispora tropica'' is an obligate marine actinomycete bacterium species. It produces salinosporamide A and salinosporamide B, potential anti-cancer agents, as well as the polycyclic macrolides The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macr ... sporolide A and B. See also * '' Salinispora arenicola'' * '' Salinispora pacifica'' References Further reading * * * External links LPSN*WORMS entry [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrolides
The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but is now used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic. Macrolides are bacteriostatic in that they suppress or inhibit bacterial growth rather than killing bacteria completely. Definition In general, any macrocyclic lactone having greater than 8-membered rings are candidates for this class. The macrocycle may contain amino nitrogen, amide nitrogen (but should be differentiated from cyclopeptides), an oxazole ring, or a thiazole ring. Benzene rings are exclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compounds With 7 Or More Rings
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrolides
The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but is now used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic. Macrolides are bacteriostatic in that they suppress or inhibit bacterial growth rather than killing bacteria completely. Definition In general, any macrocyclic lactone having greater than 8-membered rings are candidates for this class. The macrocycle may contain amino nitrogen, amide nitrogen (but should be differentiated from cyclopeptides), an oxazole ring, or a thiazole ring. Benzene rings are exclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthesis
Synthesis or synthesize may refer to: Science Chemistry and biochemistry *Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors ** Organic synthesis, the chemical synthesis of organic compounds ***Total synthesis, the complete organic synthesis of complex organic compounds, usually without the aid of biological processes ***Convergent synthesis or linear synthesis, a strategy to improve the efficiency of multi-step chemical syntheses **Dehydration synthesis, a chemical synthesis resulting in the loss of a water molecule * Biosynthesis, the creation of an organic compound in a living organism, usually aided by enzymes **Photosynthesis, a biochemical reaction using a carbon molecule to produce an organic molecule, using sunlight as a catalyst **Chemosynthesis, the synthesis of biological compounds into organic waste, using methane or an oxidized molecule as a catalyst **Amino acid synthesis, the synthesis of an amino ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-quinone
1,2-Benzoquinone, also called ''ortho''-benzoquinone, is an organic compound with formula . It is one of the two isomers of quinone, the other being 1,4-Benzoquinone, 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent compound of many derivative (chemistry), derivatives which are known. Structure The molecule has C symmetry. X-ray crystallography shows that the double bonds are localized, with alternatingly long and short C-C distances within the ring. The C=O distances of 1.21 Å are characteristic of ketones. Preparation and occurrence 1,2-Benzoquinone is produced on oxidation of catechol exposed to air in aqueous solution or by ortho oxidation of a phenol. It is a precursor to melanin. A strain of the bacterium ''Pseudomonas mendocina'' metabolism, metabolises benzoic acid, yielding 1,2-benzoquinone via catechol. References {{D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often require a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful. Often, the aim is to discover a new route of synthesis for a target molecule for which there already exist known routes. Sometimes, however, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
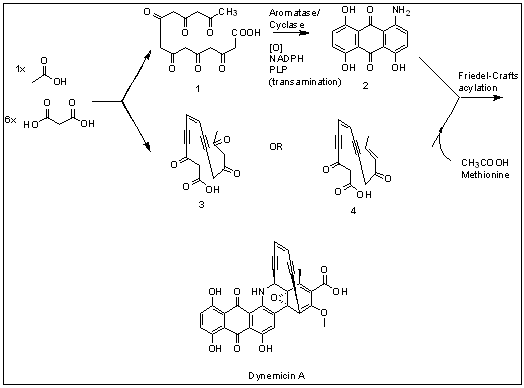
Dynemicin A
Dynemicin A is an anti-cancer enediyne drug. It displays properties which illustrate promise for cancer treatments, but still requires further research. History and background Dynemicin A was first isolated from the soil in the Gujarat State of India. It was discovered to be the natural product of the indigenous bacteria '' Micromonospora chersina''. The natural product displays a bright purple color due to the anthraquinone chromophore structure within it. Initially, this compound was isolated for its aesthetic properties as a dye until further research demonstrated its anti-cancer properties. Shortly after the compound's discovery, the Bristol-Myers Pharmaceutical Company elucidated the structure in Japan using X-ray diffraction studies of triacetyldynemicin A; a closely related compound. Synthesis The first reported chemical synthesis of dynemicin was accomplished by Myers and coworkers. Biosynthesis Dynemicin A is an antitumor natural product isolated from ''Micro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marine Bacterium
Marine prokaryotes are marine bacteria and marine archaea. They are defined by their habitat as prokaryotes that live in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. All cellular life forms can be divided into prokaryotes and eukaryotes. Eukaryotes are organisms whose cells have a nucleus enclosed within membranes, whereas prokaryotes are the organisms that do not have a nucleus enclosed within a membrane. The three-domain system of classifying life adds another division: the prokaryotes are divided into two domains of life, the microscopic bacteria and the microscopic archaea, while everything else, the eukaryotes, become the third domain. Prokaryotes play important roles in ecosystems as decomposers recycling nutrients. Some prokaryotes are pathogenic, causing disease and even death in plants and animals. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |