|
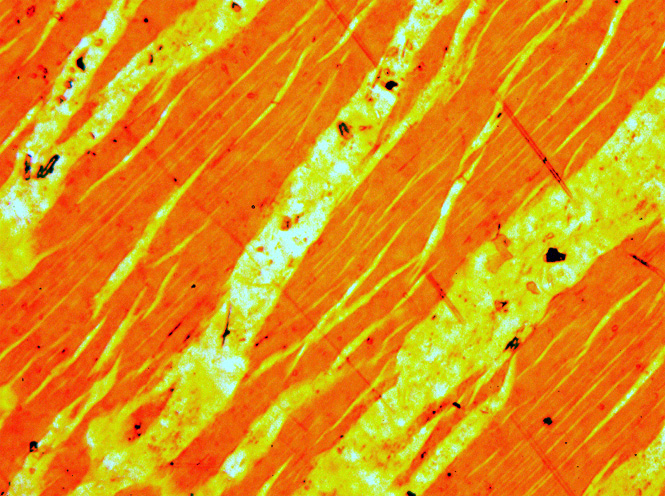
Solvus
In a physical or geochemical system, a solvus is a line (binary system) or surface (ternary system) on a phase diagram which separates a homogeneous solid solution from a field of several phases which may form by exsolution or incongruent melting. The line determines a solid solubility limit which changes as a function of temperature. It is a locus of points on the equilibrium diagram. An example is the formation of perthite when an alkali feldspar is cooled down. It defines the limit of solid solubility in an equilibrium diagram. See also *Miscibility gap A miscibility gap is a region in a phase diagram for a mixture of components where the mixture exists as two or more phases – any region of composition of mixtures where the constituents are not completely miscible. The IUPAC Gold Book defines ... References Geochemistry Petrology Materials science {{Petrology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Overview Common components of a phase diagram are ''lines of equilibrium'' or ''phase boundaries'', which refer to lines that mark conditions under which multiple phases can coexist at equilibrium. Phase transitions occur along lines of equilibrium. Metastable phases are not shown in phase diagrams as, despite their common occurrence, they are not equilibrium phases. Triple points are points on phase diagrams where lines of equilibrium intersect. Triple points mark conditions at which three different phases can coexist. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can coexist in a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Solution
A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions - ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miscibility Gap
A miscibility gap is a region in a phase diagram for a mixture of components where the mixture exists as two or more phases – any region of composition of mixtures where the constituents are not completely miscible. The IUPAC Gold Book defines ''miscibility gap'' as "Area within the coexistence curve of an isobaric phase diagram (temperature vs composition) or an isothermal phase diagram (pressure vs composition)." A miscibility gap between isostructural phases may be described as the '' solvus'', a term also used to describe the boundary on a phase diagram between a miscibility gap and other phases. Thermodynamically, miscibility gaps indicate a maximum (''e.g.'' of Gibbs energy) in the composition range. Named miscibility gaps A number of miscibility gaps in phase systems are named, including * The ''huttenlocher'' (found in bytownite Bytownite is a calcium rich member of the plagioclase solid solution series of feldspar minerals with composition between anorthite and la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to resolve include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geochemistry
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the entire Solar System, and has made important contributions to the understanding of a number of processes including mantle convection, the formation of planets and the origins of granite and basalt. It is an integrated field of chemistry and geology. History The term ''geochemistry'' was first used by the Swiss-German chemist Christian Friedrich Schönbein in 1838: "a comparative geochemistry ought to be launched, before geognosy can become geology, and before the mystery of the genesis of our planets and their inorganic matter may be revealed." However, for the rest of the century the more common term was "chemical geology", and there was little contact between geologists and chemists. Geochemistry emerged as a separate discipline af ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incongruent Melting
Incongruent melting occurs when a solid substance does not melt uniformly, so that the chemical composition of the resulting liquid is not the same as that of the original solid. During incongruent melting a new solid of different composition forms. For example, melting of orthoclase (KAlSi3O8) produces leucite (KAlSi2O6) in addition to a melt. The melt produced is richer in silica (SiO2). The proportions of leucite and melt created can be recombined to yield the bulk composition of the starting feldspar. Another mineral that can melt incongruently is enstatite (Mg2Si2O6), which produces forsterite (Mg2SiO4) in addition to a melt richer in SiO2 when melting at low pressure. Enstatite melts congruently at higher pressures between 2.5 and 5.5 kilobars. See also * Congruent melting * Incongruent transition * Phase diagram A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perthite
Perthite is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na-rich feldspar domains se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldspars and the ''alkali'' (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust, and 41% of the Earth's continental crust by weight. Feldspars crystalize from magma as both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks. Compositions The feldspar group of minerals consists of tectosilicates, silicate minerals in which silicon ions are linked by shared oxygen ions to form a three-dimensional network. Compositions of major elements in common feldspars can be expressed in terms of three endmembers: * potassium feldspar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petrology
Petrology () is the branch of geology that studies rocks and the conditions under which they form. Petrology has three subdivisions: igneous, metamorphic, and sedimentary petrology. Igneous and metamorphic petrology are commonly taught together because they both contain heavy use of chemistry, chemical methods, and phase diagrams. Sedimentary petrology is, on the other hand, commonly taught together with stratigraphy because it deals with the processes that form sedimentary rock. Background Lithology was once approximately synonymous with petrography, but in current usage, lithology focuses on macroscopic hand-sample or outcrop-scale description of rocks while petrography is the speciality that deals with microscopic details. In the petroleum industry, lithology, or more specifically mud logging, is the graphic representation of geological formations being drilled through and drawn on a log called a mud log. As the cuttings are circulated out of the borehole, they are s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |