|
Silver Glance
In mineralogy, argentite (from the Latin ''argentum'', silver) is cubic silver sulfide (Ag2S), which can only exist at temperatures above 173 °C, 177 °C or 179 °C. When it cools to ordinary temperatures it turns into its monoclinic polymorph, acanthite. The International Mineralogical Association has decided to reject argentite as a proper mineral. The name "argentite" sometimes also refers to pseudomorphs of argentite: specimens of acanthite which still display some of the outward signs of the cubic crystal form, even though their actual crystal structure is monoclinic due to the lower temperature. This form of acanthite is occasionally found as uneven cubes and octahedra, but more often as dendritic or earthy masses, with a blackish lead-grey color and metallic luster. Argentite belongs to the galena group. Cleavage, which is so prominent a feature in galena, here presents only in traces. The mineral is perfectly sectile and has a shining streak; hardness 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argentite
In mineralogy, argentite (from the Latin ''argentum'', silver) is cubic silver sulfide (Ag2S), which can only exist at temperatures above 173 °C, 177 °C or 179 °C. When it cools to ordinary temperatures it turns into its monoclinic polymorph, acanthite. The International Mineralogical Association has decided to reject argentite as a proper mineral. The name "argentite" sometimes also refers to pseudomorphs of argentite: specimens of acanthite which still display some of the outward signs of the cubic crystal form, even though their actual crystal structure is monoclinic due to the lower temperature. This form of acanthite is occasionally found as uneven cubes and octahedra, but more often as dendritic or earthy masses, with a blackish lead-grey color and metallic luster. Argentite belongs to the galena group. Cleavage, which is so prominent a feature in galena, here presents only in traces. The mineral is perfectly sectile and has a shining streak; hardness 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver. Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms. It is often associated with the minerals sphalerite, calcite and fluorite. Occurrence Galena is the main ore of lead, used since ancient times, since lead can be smelted from galena in an ordinary wood fire. Galena typically is found in hydrothermal veins in association with sphalerite, marcasite, chalcopyrite, cerussite, anglesite, dolomite, calcite, quartz, barite, and fluorite. It is also found in association with sphalerite in low-temperature lead-zinc deposits within limestone beds. Minor amounts are found in contact metamorphic zones, in pegmatites, and disseminated in sedimentary rock. In some deposits the galena contains up to 0.5% silver, a byproduct that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jalpaite
Jalpaite is a rare copper silver sulfide mineral with formula Ag3CuS2. It was first described in 1858 for an occurrence in the Leonora Mine, Jalpa, Zacatecas, Mexico and named for the locality. It occurs in low temperature hydrothermal veins at temperatures less than . Associated minerals include acanthite, mckinstryite, galena, sphalerite, pyrite, chalcopyrite, stromeyerite, polybasite, pearceite, tetrahedrite–tennantite Tennantite is a copper arsenic sulfosalt mineral with an ideal formula . Due to variable substitution of the copper by iron and zinc the formula is . It is gray-black, steel-gray, iron-gray or black in color. A closely related mineral, tetrahedri ... and native silver. References Sulfide minerals Copper(I) minerals Silver minerals Tetragonal minerals Minerals in space group 141 {{sulfide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jalpa, Zacatecas
Jalpa is a town located in the Mexican state of Zacatecas, close to the border with Jalisco and Aguascalientes and about a two hours drive south of the capital city, Zacatecas. Jalpa is a colonial-style city, with cobble stone streets, narrow walkways, two main churches: El Señor de Jalpa and La Parroquia de San Antonio, and two plazas. Jalpa was modeled by the French in the 19th century. In the middle of the plaza is a kiosk which remains in good shape today, after hundreds of years. Most houses are painted in bright colors just as in colonial times. The houses are made of adobe and share common walls and most have flat roofs. The original indigenous natives were the Caxcan, Chichimeca and Huichol people. History Jalpa was founded in 1532 by Spanish explorers in search of gold and silver. Jalpa was spelled "Xalpa" by its native Caxcan, Chichimeca, and Huichol people. Conquered by the Spanish, the Indian population intermixed with Spanish and other European peoples to form today's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nevada
Nevada ( ; ) is a U.S. state, state in the Western United States, Western region of the United States. It is bordered by Oregon to the northwest, Idaho to the northeast, California to the west, Arizona to the southeast, and Utah to the east. Nevada is the List of U.S. states and territories by area, 7th-most extensive, the List of U.S. states and territories by population, 32nd-most populous, and the List of U.S. states and territories by population density, 9th-least densely populated of the U.S. states. Nearly three-quarters of Nevada's people live in Clark County, Nevada, Clark County, which contains the Las Vegas–Paradise, NV MSA, Las Vegas–Paradise metropolitan area, including three of the state's four largest incorporated cities. Nevada's capital is Carson City, Nevada, Carson City. Las Vegas is the largest city in the state. Nevada is officially known as the "Silver State" because of the importance of silver to its history and economy. It is also known as the "Battle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
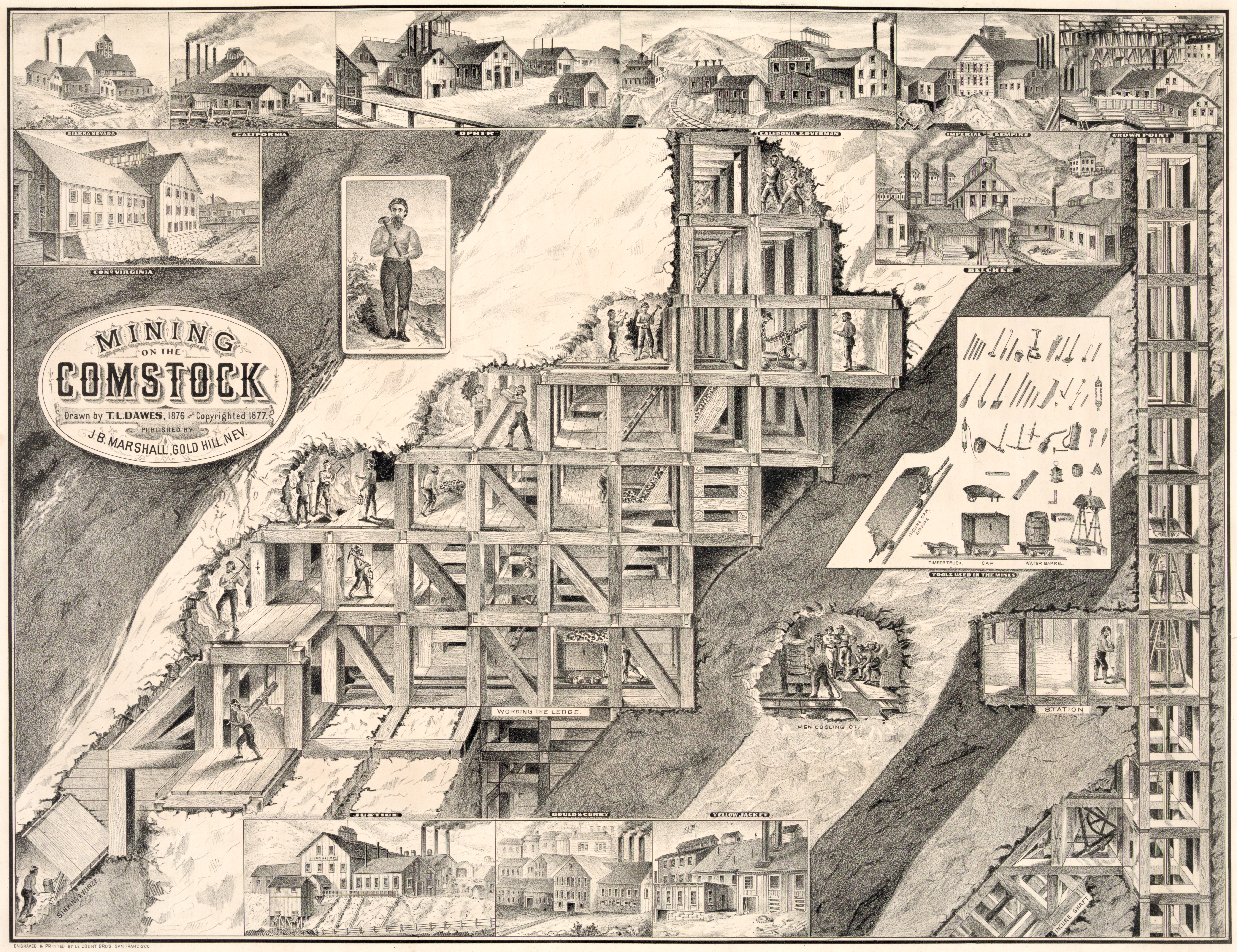
Comstock Lode
The Comstock Lode is a lode of silver ore located under the eastern slope of Mount Davidson, a peak in the Virginia Range in Virginia City, Nevada (then western Utah Territory), which was the first major discovery of silver ore in the United States and named after American miner Henry Comstock. After the discovery was made public in 1859, it sparked a silver rush of prospectors to the area, scrambling to stake their claims. The discovery caused considerable excitement in California and throughout the United States, the greatest since the California Gold Rush in 1849. Mining camps soon thrived in the vicinity, which became bustling commercial centers, including Virginia City and Gold Hill. The Comstock Lode is notable not just for the immense fortunes it generated and the large role those fortunes had in the growth of Nevada and San Francisco, but also for the advances in mining technology that it spurred, such as square set timbering and the Washoe process for extracting silv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mexico
Mexico (Spanish: México), officially the United Mexican States, is a country in the southern portion of North America. It is bordered to the north by the United States; to the south and west by the Pacific Ocean; to the southeast by Guatemala, Belize, and the Caribbean Sea; and to the east by the Gulf of Mexico. Mexico covers ,Mexico ''''. . making it the world's 13th-largest country by are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (often abbreviated r.d. or RD) is often preferred in scientific usage, whereas the term "specific gravity" is deprecation, deprecated. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. A substance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard metals such as titanium and beryllium are harder than soft metals such as sodium and metallic tin, or wood and common plastics. Macroscopic hardness is generally characterized by strong intermolecular bonds, but the behavior of solid materials under force is complex; therefore, hardness can be measured in different ways, such as scratch hardness, indentation hardness, and rebound hardness. Hardness is dependent on ductility, elastic stiffness, plasticity, strain, strength, toughness, viscoelasticity, and viscosity. Common examples of hard matter are ceramics, concrete, certain metals, and superhard materials, which can be contrasted with soft matter. Measuring hardness There are three main types of hardness measurements: ''scratch' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sectility
Sectility is the ability of a mineral to be cut into thin pieces with a knife. Minerals that are not sectile will be broken into rougher pieces when cut. Metals and paper are sectile. Sectility can be used to distinguish minerals of similar appearance, and is a form of tenacity. For example, gold is sectile but pyrite ("fool's gold") is not. Sectility in metals is a result of metallic bonding, where valence (bonding) electrons are delocalized and can flow freely between atoms, rather than being shared between specific pairs or groups of atoms, as in covalent bond A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...ing. References Mineralogy {{Geology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cleavage (crystal)
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage."Hurlbut, Cornelius S.; Klein, Cornelis, 1985, '' Manual of Mineralogy'', 20th ed., Wiley, The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica." Diamond and graphite provide examples of cleavage. Both are composed solely of a single element, carbon. But in diamond, each carbon atom is bonded to four others in a tetrahedral pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedron
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. A regular octahedron is the dual polyhedron of a cube. It is a rectified tetrahedron. It is a square bipyramid in any of three orthogonal orientations. It is also a triangular antiprism in any of four orientations. An octahedron is the three-dimensional case of the more general concept of a cross polytope. A regular octahedron is a 3-ball in the Manhattan () metric. Regular octahedron Dimensions If the edge length of a regular octahedron is ''a'', the radius of a circumscribed sphere (one that touches the octahedron at all vertices) is :r_u = \frac a \approx 0.707 \cdot a and the radius of an inscribed sphere (tangent to each of the octahedron's faces) is :r_i = \frac a \approx 0.408\cdot a while the midradius, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)