|
Serum Bilirubin
Bilirubin (BR) ( Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the destruction of aged or abnormal red blood cells. In the first step of bilirubin synthesis, the heme molecule is stripped from the hemoglobin molecule. Heme then passes through various processes of porphyrin catabolism, which varies according to the region of the body in which the breakdown occurs. For example, the molecules excreted in the urine differ from those in the feces. The production of biliverdin from heme is the first major step in the catabolic pathway, after which the enzyme biliverdin reductase performs the second step, producing bilirubin from biliverdin.Boron W, Boulpaep E. Medical Physiology: a cellular and molecular approach, 2005. 984–986. Elsevier Saunders, United States. Ultimately, bilirubin is broken down ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latin
Latin (, or , ) is a classical language belonging to the Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power of the Roman Republic it became the dominant language in the Italian region and subsequently throughout the Roman Empire. Even after the fall of Western Rome, Latin remained the common language of international communication, science, scholarship and academia in Europe until well into the 18th century, when other regional vernaculars (including its own descendants, the Romance languages) supplanted it in common academic and political usage, and it eventually became a dead language in the modern linguistic definition. Latin is a highly inflected language, with three distinct genders (masculine, feminine, and neuter), six or seven noun cases (nominative, accusative, genitive, dative, ablative, and vocative), five declensions, four verb conjuga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urobilin
Urobilin or urochrome is the chemical primarily responsible for the yellow color of urine. It is a linear tetrapyrrole compound that, along with the related colorless compound urobilinogen, are degradation products of the cyclic tetrapyrrole heme. Metabolism Urobilin is generated from the degradation of heme, which is first degraded through biliverdin to bilirubin. Bilirubin is then excreted as bile, which is further degraded by microbes present in the large intestine to urobilinogen. Some of this remains in the large intestine, and its conversion to stercobilin gives feces their brown color. Some is reabsorbed into the bloodstream and then delivered to kidney. When urobilinogen is exposed to air, it is oxidized to urobilin, giving urine its yellow color. Importance Many urine tests (urinalysis) monitor the amount of urobilin in urine, as its levels can give insight on the effectiveness of urinary tract function. Normally, urine would appear as either light yellow or colorless. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azobilirubin
Azobilirubin is a coloured compound formed by the oxidation of diazotized sulfanilic acid with bilirubin in the van den Bergh reaction. The quantity of bilirubin in patients with jaundice can be determined by the formation of azobilirubin in the presence of methanol Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a .... The Van den Bergh chemical reaction which is used to measure bilirubin levels, couples bilirubin with diazotized sulfanilic acid. This reaction produced azo pigments, or azobilirubin. The presence of azobilirubin is best indicated by the emergence of a pink-purple color. The intensity of the color will also indicate how much bilirubin is in the blood. Color markers and indicators can be changed. Adding alkaline tartrate can make the purple azobilirubin into a blue azobi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug-induced Liver Injury
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval. The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses (e.g. paracetamol) and sometimes even when introduced within therapeutic ranges (e.g. halothane), may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., microcystins), and herbal remedies (two prominent examples being kava, mechanism unknown, and comfrey, through its pyrrolizidine alkaloid content) can also induce hepatotoxicity. Chemicals that cause liver injury are called hepatotoxins. More than 900 drugs have been implicated in causing liver injury (see LiverTox, externa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hy's Law
Hy's law is a rule of thumb that a patient is at high risk of a fatal drug-induced liver injury if given a medication that causes hepatocellular injury (not Hepatobiliary injury) with jaundice. The law is based on observations by Hy Zimmerman, a major scholar of drug-induced liver injury.United States Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER)Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation, Final, July 2009/ref> Some have suggested the principle be called a hypothesis or observation. Hy's Law cases have three components: * The drug causes hepatocellular injury, generally defined as an elevated ALT or AST by 3-fold or greater above the upper limit of normal. Often with aminotransferases much greater (5-10x) the upper limit of normal. * Among subjects showing such aminotransferase elevations, they also have elevation of thei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
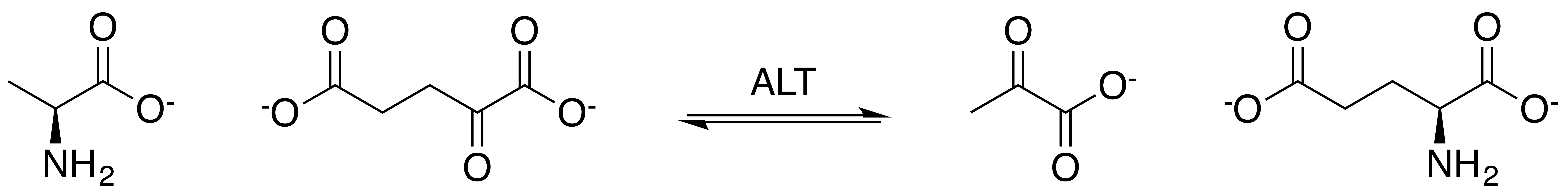
Alanine Aminotransferase
Alanine transaminase (ALT) is a transaminase enzyme (). It is also called alanine aminotransferase (ALT or ALAT) and was formerly called serum glutamate-pyruvate transaminase or serum glutamic-pyruvic transaminase (SGPT) and was first characterized in the mid-1950s by Arthur Karmen and colleagues. ALT is found in plasma and in various body tissues but is most common in the liver. It catalyzes the two parts of the alanine cycle. Serum ALT level, serum AST (aspartate transaminase) level, and their ratio (AST/ALT ratio) are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels. The half-life of ALT in the circulation approximates 47 hours. Aminotransferase is cleared by sinusoidal cells in the liver. Function ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate, the products of this reversible transamination reaction being pyruvate and L-glutamate. : L-alanine + α-ketoglutarate ⇌ pyruvate + L-glutamate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme Breakdown
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver. In biochemical terms, heme is a coordination complex "consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands." The definition is loose, and many depictions omit the axial ligands. Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase. The word ''haem'' is derived from Greek ''haima'' meaning "blood". Function Hemoproteins have diverse biological functions inc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phototherapy
Light therapy, also called phototherapy or bright light therapy is intentional daily exposure to direct sunlight or similar-intensity artificial light in order to treat medical disorders, especially seasonal affective disorder (SAD) and circadian rhythm sleep-wake disorders. Treating skin conditions such as neurodermatitis, psoriasis, acne vulgaris, and eczema with ultraviolet light is called ultraviolet light therapy. Medical uses Nutrient deficiency Vitamin D deficiency Exposure to light at specific wavelengths of Ultraviolet B (abbreviated as UV-B or UVB) enables the body to produce vitamin D to treat vitamin D deficiency. Skin conditions Light therapy treatments for the skin usually involve exposure to ultraviolet light. The exposures can be to a small area of the skin or over the whole body surface, as in a tanning bed. The most common treatment is with narrowband UVB, which has a wavelength of approximately 311–313 nanometers. Full body phototherapy can be de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytochrome
Phytochromes are a class of photoreceptor in plants, bacteria and fungi used to detect light. They are sensitive to light in the red and far-red region of the visible spectrum and can be classed as either Type I, which are activated by far-red light, or Type II that are activated by red light. Recent advances have suggested that phytochromes also act as temperature sensors, as warmer temperatures enhance their de-activation. All of these factors contribute to the plant's ability to germinate. Phytochromes control many aspects of plant development. They regulate the germination of seeds (photoblasty), the synthesis of chlorophyll, the elongation of seedlings, the size, shape and number and movement of leaves and the timing of flowering in adult plants. Phytochromes are widely expressed across many tissues and developmental stages. Other plant photoreceptors include cryptochromes and phototropins, which respond to blue and ultraviolet-A light and UVR8, which is sensitive to u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phycobilin
Phycobilins (from Greek: '' (phykos)'' meaning "alga", and from Latin: ''bilis'' meaning "bile") are light-capturing bilins found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads (though not in green algae and plants). Most of their molecules consist of a chromophore which makes them coloured. They are unique among the photosynthetic pigments in that they are bonded to certain water-soluble proteins, known as phycobiliproteins. Phycobiliproteins then pass the light energy to chlorophylls for photosynthesis. The phycobilins are especially efficient at absorbing red, orange, yellow, and green light, wavelengths that are not well absorbed by chlorophyll ''a''. Organisms growing in shallow waters tend to contain phycobilins that can capture yellow/red light, while those at greater depth often contain more of the phycobilins that can capture green light, which is relatively more abundant there. The phycobilins fluoresce at a particular wavel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compounds. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli. Economic impact In 2006, around 7.4 million tons of inorganic, organic, and special pigments were marketed worldwide. Estimated at around US$14.86 billion in 2018 and will rise at over 4.9% CAGR from 2019 to 2026. The global demand for pigments was roughly US$20.5 billion in 2009. According to an April 2018 report by ''Bloomberg Businessweek'', the estimated value of the pigment industry globally is $30 billion. The value of titanium dioxide – used to enhance the white brightness of many products – was placed at $13.2 billion per year, while the color Ferrari red is valued at $300 million each year. Physical principles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |