|
Seneca Glass Company
Seneca Glass Company was a glass manufacturer that began in Fostoria, Ohio, in1891. At one time it was the largest manufacturer of blown tumblers (drinking glasses) in the United States. The company was also known for its high-quality lead (crystal) stemware, which was hand-made for nearly a century. Customers included Eleanor Roosevelt and Lyndon B. Johnson, and retailers such as Marshall Field and Company, Neiman Marcus, and Tiffany's. The company took possession of its Fostoria plant on January 1, 1892, after it was vacated by the Fostoria Glass Company. Otto Jaeger was the first president of Seneca Glass Company, and he had been part of the Fostoria Glass Company management team. Like Jaeger, many of the new company's original leaders were German craftsmen experienced in glassmaking. In addition to being investors in the company, these craftsmen worked in the plant. In 1896, the firm moved to Morgantown, West Virginia, and continued to produce high-quality decorated glasswar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
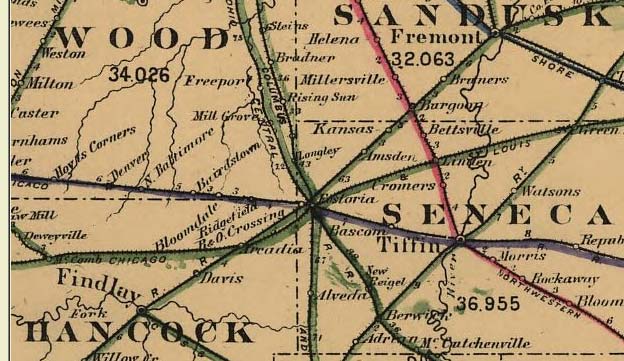
Fostoria, Ohio
Fostoria (, ) is a city located at the convergence of Hancock, Seneca, and Wood counties in the northwestern part of the U.S. state of Ohio. It is approximately south of Toledo and north of Columbus. The city is known for its railroads, as approximately 100 trains pass through the city each day. The city is often visited by railfans, and a railroad viewing park, constructed in 2013 (dedicated 14 November 2013) hosts many railfans every day in a purpose-built viewing platform. Fostoria was also the home for over a dozen glass factories during the end of the 19th century. The glass factories were established in Fostoria because of the discovery of natural gas in the area. As the gas supply became depleted, many of the factories closed or moved—including the Fostoria Glass Company. Fostoria's most famous citizen is Charles Foster (son of the man who helped establish Fostoria), who became governor of Ohio. The community grew substantially during the end of the 19th century ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germans
, native_name_lang = de , region1 = , pop1 = 72,650,269 , region2 = , pop2 = 534,000 , region3 = , pop3 = 157,000 3,322,405 , region4 = , pop4 = 21,000 3,000,000 , region5 = , pop5 = 125,000 982,226 , region6 = , pop6 = 900,000 , region7 = , pop7 = 142,000 840,000 , region8 = , pop8 = 9,000 500,000 , region9 = , pop9 = 357,000 , region10 = , pop10 = 310,000 , region11 = , pop11 = 36,000 250,000 , region12 = , pop12 = 25,000 200,000 , region13 = , pop13 = 233,000 , region14 = , pop14 = 211,000 , region15 = , pop15 = 203,000 , region16 = , pop16 = 201,000 , region17 = , pop17 = 101,000 148,00 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molding (process)
Molding (American English) or moulding (British and Commonwealth English; see spelling differences) is the process of manufacturing by shaping liquid or pliable raw material using a rigid frame called a mold or matrix. This itself may have been made using a pattern or model of the final object. A mold or mould is a hollowed-out block that is filled with a liquid or pliable material such as plastic, glass, metal, or ceramic raw material. The liquid hardens or sets inside the mold, adopting its shape. A mold is a counterpart to a cast. The very common bi-valve molding process uses two molds, one for each half of the object. Articulated molds have multiple pieces that come together to form the complete mold, and then disassemble to release the finished casting; they are expensive, but necessary when the casting shape has complex overhangs. Piece-molding uses a number of different molds, each creating a section of a complicated object. This is generally only used for larger a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressed Glass
Pressed glass (or pattern glass) is a form of made by pressing molten glass into a using a . It was first patented by American inventor John P. Bakewell in 1825 to make knobs for furniture. The technique was developed in the from the 1820s an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glassblowing
Glassblowing is a glassforming technique that involves inflating molten glass into a bubble (or parison) with the aid of a Blowpipe (tool), blowpipe (or blow tube). A person who blows glass is called a ''glassblower'', ''glassmith'', or ''gaffer''. A ''lampworking, lampworker'' (often also called a glassblower or glassworker) manipulates glass with the use of a torch on a smaller scale, such as in producing precision laboratory glassware out of borosilicate glass. Technology Principles As a novel glass forming technique created in the middle of the 1st century BC, glassblowing exploited a working property of glass that was previously unknown to glassworkers; inflation, which is the expansion of a molten blob of glass by introducing a small amount of air into it. That is based on the liquid structure of glass where the atoms are held together by strong chemical bonds in a disordered and random network,Frank, S 1982. Glass and Archaeology. Academic Press: London. Freestone, I. (1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plate Glass
Plate glass, flat glass or sheet glass is a type of glass, initially produced in plane form, commonly used for windows, glass doors, transparent walls, and windscreens. For modern architectural and automotive applications, the flat glass is sometimes bent after production of the plane sheet. Flat glass stands in contrast to ''container glass'' (used for bottles, jars, cups) and '' glass fibre'' (used for thermal insulation, in fibreglass composites, and for optical communication). Flat glass has a higher magnesium oxide and sodium oxide content than container glass, and a lower silica, calcium oxide, and aluminium oxide content."High temperature glass melt property database for process modeling"; Eds.: Thomas P. Seward III and Terese Vascott; The American Ceramic Society, Westerville, Ohio, 2005, From the lower soluble oxide content comes the better chemical durability of container glass against water, which is required especially for storage of beverages and food. Most f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lime (material)
Lime is a calcium-containing inorganic material composed primarily of oxides and hydroxide, usually calcium oxide and/or calcium hydroxide. It is also the name for calcium oxide which occurs as a product of coal-seam fires and in altered limestone xenoliths in volcanic ejecta. The International Mineralogical Association recognizes lime as a mineral with the chemical formula of CaO. The word ''lime'' originates with its earliest use as building mortar and has the sense of ''sticking or adhering''. These materials are still used in large quantities as building and engineering materials (including limestone products, cement, concrete, and mortar), as chemical feedstocks, and for sugar refining, among other uses. Lime industries and the use of many of the resulting products date from prehistoric times in both the Old World and the New World. Lime is used extensively for wastewater treatment with ferrous sulfate. The rocks and minerals from which these materials are derived, typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead(II,IV) Oxide
Lead(II,IV) oxide, also called red lead or minium, is the inorganic compound with the formula Pb3O4. A bright red or orange solid, it is used as pigment, in the manufacture of batteries, and rustproof primer paints. It is an example of a mixed valence compound, being composed of both Pb(II) and Pb(IV) in the ratio of two to one. Structure Lead(II,IV) oxide has a tetragonal crystal structure at room temperature, which then transforms to an orthorhombic ( Pearson symbol ''oP''28, Space group Pbam, No. 55) form at temperature . This phase transition only changes the symmetry of the crystal and slightly modifies the interatomic distances and angles. File:Red-lead-unit-cell-3D-balls.png, Unit cell of tetragonal Pb3O4(Key: Pb O) File:Red-lead-3D-balls.png, Part of tetragonal red lead's crystal structure Preparation Lead(II,IV) oxide is prepared by calcination of lead(II) oxide (PbO; also called litharge) in air at about 450–480 °C: :6 PbO + O2 -> 2 Pb3O4 The res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potash
Potash () includes various mined and manufactured salts that contain potassium in water-soluble form.Potash USGS 2008 Minerals Yearbook The name derives from ''pot ash'', plant ashes or soaked in water in a pot, the primary means of manufacturing potash before the . The word '''' is derived from ''potash''. Potash is produced worldwide in amounts exceeding 90 million |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dioxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Batch Calculation
Glass batch calculation or glass batching is used to determine the correct mix of raw materials (batch) for a glass melt. Principle The raw materials mixture for glass melting is termed "batch". The batch must be measured properly to achieve a given, desired glass formulation. This batch calculation is based on the common linear regression equation: N_B = (B^T\cdot B)^\cdot B^T \cdot N_G with NB and NG being the molarities 1-column matrices of the batch and glass components respectively, and B being the batching matrix. The symbol "T" stands for the matrix transpose operation, "−1" indicates matrix inversion, and the sign "·" means the scalar product. From the molarities matrices N, percentages by weight (wt%) can easily be derived using the appropriate molar masses. Example calculation An example batch calculation may be demonstrated here. The desired glass composition in wt% is: 67 SiO2, 12 Na2O, 10 CaO, 5 Al2O3, 1 K2O, 2 MgO, 3 B2O3, and as raw materials are used sand, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |