|
Sea Ice Microbial Communities
Sea Ice Microbial Communities (SIMCO) refer to groups of microorganisms living within and at the interfaces of sea ice at the poles. The ice matrix they inhabit has strong vertical gradients of salinity, light, temperature and nutrients. Sea ice chemistry is most influenced by the salinity of the brine which affects the pH and the concentration of dissolved nutrients and gases. The brine formed during the melting sea ice creates pores and channels in the sea ice in which these microbes can live. As a result of these gradients and dynamic conditions, a higher abundance of microbes are found in the lower layer of the ice, although some are found in the middle and upper layers. Despite this extreme variability in environmental conditions, the Taxonomic rank, taxonomical community composition tends to remain consistent throughout the year, until the ice melts. Much of the knowledge concerning the community diversity of the sea ice is known through Genetic analysis, genetic analyses an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
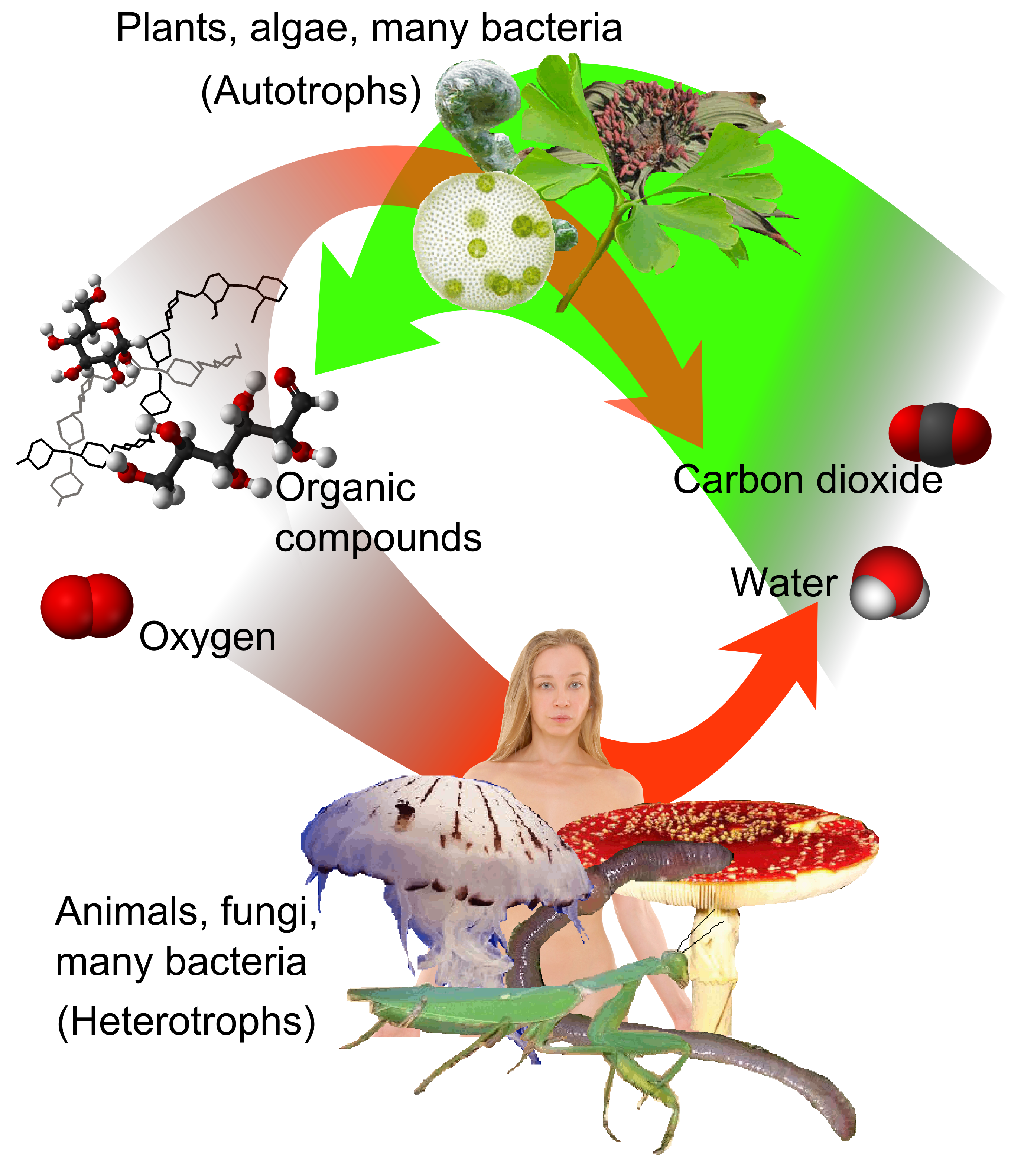
Primary Production
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through chemosynthesis, which uses the oxidation or reduction of inorganic chemical compounds as its source of energy. Almost all life on Earth relies directly or indirectly on primary production. The organisms responsible for primary production are known as ''primary producers'' or autotrophs, and form the base of the food chain. In terrestrial ecoregions, these are mainly plants, while in aquatic ecoregions algae predominate in this role. Ecologists distinguish primary production as either ''net'' or ''gross'', the former accounting for losses to processes such as cellular respiration, the latter not. Overview Primary production is the production of chemical energy in organic compounds by living organisms. The main source of this energy is su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extreme Environment
An extreme environment is a habitat that is considered very hard to survive in due to its considerably extreme conditions such as temperature, accessibility to different energy sources or under high pressure. For an area to be considered an extreme environment, it must contain certain conditions and aspects that are considered very hard for other life forms to survive. Pressure conditions may be extremely high or low; high or low content of oxygen or carbon dioxide in the atmosphere; high levels of radiation, acidity, or alkalinity; absence of water; water containing a high concentration of salt; the presence of sulphur, petroleum, and other toxic substances. Examples of extreme environments include the geographical poles, very arid deserts, volcanoes, deep ocean trenches, upper atmosphere, outer space, and the environments of every planet in the Solar System except the Earth. Any organisms living in these conditions are often very well adapted to their living circumstances, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperoxia
Hyperoxia occurs when cells, tissues and organs are exposed to an excess supply of oxygen (O2) or higher than normal partial pressure of oxygen. In medicine, it refers to excessive oxygen in the lungs or other body tissues, which can be caused by breathing air at pressures greater than normal atmospheric pressure, or breathing gas mixtures whenever the alveolar oxygen partial pressure is greater than that due to breathing air at normal atmospheric pressure, which can be a consequence of high oxygen fraction, high ambient pressure or a combination of ambient pressure and oxygen fraction resulting in an oxygen partial pressure significantly higher than that of sea level air. The body is tolerant of some deviation from normal inspired oxygen partial pressure, but a sufficiently elevated level of hyperoxia can lead to oxygen toxicity over time, with the mechanism related to the partial pressure, and the severity related to the dose. Hyperoxia is the opposite of hypoxia; hyperoxia r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Carbon Compound
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings. Allotropes of carbon The known inorganic chemistry of the allotropes of carbon (diamond, graphite, and the fullerenes) blossomed with the discovery of buckminsterfullerene in 1985, as additional fullerenes and their various derivatives were discovered. One such class of derivatives is inclusion compounds, in which an ion is enclosed by the all-carbon shell of the fullerene. This inclusion is denoted by the "@" symbol in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water – hence the name ''photosynthesis'', from the Greek ''phōs'' (), "light", and ''synthesis'' (), "putting together". Most plants, algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth. Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centers that contain green chlorophyll (and other colored) pigments/chromoph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotroph
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but not producers. Living organisms that are heterotrophic include all animals and fungi, some bacteria and protists, and many parasitic plants. The term heterotroph arose in microbiology in 1946 as part of a classification of microorganisms based on their type of nutrition. The term is now used in many fields, such as ecology in describing the food chain. Heterotrophs may be subdivided according to their energy source. If the heterotroph uses chemical energy, it is a chemoheterotroph (e.g., humans and mushrooms). If it uses light for energy, then it is a photoheterotroph (e.g., green non-sulfur bacteria). Heterotrophs represent one of the two mechanisms of nutrition (trophic levels), the other being autotrophs (''auto'' = self, ''troph'' = ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoxia (environmental)
Hypoxia refers to low oxygen conditions. Normally, 20.9% of the gas in the atmosphere is oxygen. The partial pressure of oxygen in the atmosphere is 20.9% of the total barometric pressure. In water, oxygen levels are much lower, approximately 7 ppm or 0.0007% in good quality water, and fluctuate locally depending on the presence of photosynthetic organisms and relative distance to the surface (if there is more oxygen in the air, it will diffuse across the partial pressure gradient). Atmospheric hypoxia Atmospheric hypoxia occurs naturally at high altitudes. Total atmospheric pressure decreases as altitude increases, causing a lower partial pressure of oxygen, which is defined as hypobaric hypoxia. Oxygen remains at 20.9% of the total gas mixture, differing from hypoxic hypoxia, where the percentage of oxygen in the air (or blood) is decreased. This is common in the sealed burrows of some subterranean animals, such as blesmols. Atmospheric hypoxia is also the basis of altitude tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen Gas
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frost Flower (sea Ice)
Frost flowers are ice crystals commonly found growing on young sea ice and thin lake ice in cold, calm conditions. The ice crystals are similar to hoar frost, and are commonly seen to grow in patches around 3–4 cm in diameter. Frost flowers growing on sea ice have extremely high salinities and concentrations of other sea water chemicals and, because of their high surface area, are efficient releasers of these chemicals into the atmosphere. Formation Frost flowers are formed on new sea ice, on the open water leads (where the existing ice has split open) when the atmosphere is much colder than the underlying ice. Open water leads are formed by the winds, tides, and currents. These leads expose water near 0 °C to much colder air, which results in the rapid formation of ice. With the formation and growth of ice, salt is simultaneously pushed out, back into the ocean due to gravity (gravity drainage) as well as outward which forms brine channels extending to the surface. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |