|
Scandium-44
Scandium-44 (44Sc) is a radioactive isotope of scandium that decays by positron emission to stable 44Ca with a half-life of 4.042 hours. 44Sc can be obtained as a daughter radionuclide of long-lived 44Ti (t1/2 60.4 a) from 44Ti /44Sc generator or can be produced by nuclear reaction 44Ca ( p, n)44Sc in small cyclotrons. This isotope is of potential interest for clinical PET imaging. Applications Titanium-44 generation 44Ti with its long half-life of ca. 60 years provides a cyclotron-independent source of 44Sc for several decades. 44Ti is obtained in the nuclear reaction 45Sc(p,2n)44Ti and it transforms directly into the ground state of 44Sc via electron capture, emitting low energetic photons at 67.9 keV and 78.3 keV. The 44Ti /44Sc generator represents a secular equilibrium system with a half-life ratio between parent and daughter of ca. 130 000. Consequently, 50% of saturation activity is generated every 3.97 hours, and identical 44Sc batch activities very close to saturat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Scandium
Naturally occurring scandium (21Sc) is composed of one stable isotope, 45Sc. Twenty-five radioisotopes have been characterized, with the most stable being 46Sc with a half-life of 83.8 days, 47Sc with a half-life of 3.35 days, and 48Sc with a half-life of 43.7 hours and 44Sc with a half-life of 3.97 hours. All the remaining isotopes have half-lives that are less than four hours, and the majority of these have half-lives that are less than two minutes, the least stable being proton unbound 39Sc with a half-life shorter than 300 nanoseconds. This element also has 13 meta states with the most stable being 44m2Sc (t1/2 58.6 h). The isotopes of scandium range in atomic weight from 38 u (36Sc) to 62 u (62Sc). The primary decay mode at masses lower than the only stable isotope, 45Sc, is beta-plus or electron capture, and the primary mode at masses above it is beta-minus. The primary decay products at atomic weights below 45Sc are calcium isotopes and the primary products from hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Scandium
Naturally occurring scandium (21Sc) is composed of one stable isotope, 45Sc. Twenty-five radioisotopes have been characterized, with the most stable being 46Sc with a half-life of 83.8 days, 47Sc with a half-life of 3.35 days, and 48Sc with a half-life of 43.7 hours and 44Sc with a half-life of 3.97 hours. All the remaining isotopes have half-lives that are less than four hours, and the majority of these have half-lives that are less than two minutes, the least stable being proton unbound 39Sc with a half-life shorter than 300 nanoseconds. This element also has 13 meta states with the most stable being 44m2Sc (t1/2 58.6 h). The isotopes of scandium range in atomic weight from 38 u (36Sc) to 62 u (62Sc). The primary decay mode at masses lower than the only stable isotope, 45Sc, is beta-plus or electron capture, and the primary mode at masses above it is beta-minus. The primary decay products at atomic weights below 45Sc are calcium isotopes and the primary products from hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Radioisotope
A trace radioisotope is a radioisotope that occurs naturally in trace amounts (i.e. extremely small). Generally speaking, trace radioisotopes have half-lives that are short in comparison with the age of the Earth, since primordial nuclides tend to occur in larger than trace amounts. Trace radioisotopes are therefore present only because they are continually produced on Earth by natural processes. Natural processes which produce trace radioisotopes include cosmic ray bombardment of stable nuclides, ordinary alpha and beta decay of the long-lived heavy nuclides, thorium-232, uranium-238, and uranium-235, spontaneous fission of uranium-238, and nuclear transmutation reactions induced by natural radioactivity, such as the production of plutonium-239 and uranium-236 from neutron capture by natural uranium. Elements The elements that occur on Earth only in traces are listed below. Isotopes of other elements (not exhaustive): *Tritium * Beryllium-7 *Beryllium-10 *Carbon-14 *Fluorin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron Emission
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (). Positron emission is mediated by the weak force. The positron is a type of beta particle (β+), the other beta particle being the electron (β−) emitted from the β− decay of a nucleus. An example of positron emission (β+ decay) is shown with magnesium-23 decaying into sodium-23: : → + + Because positron emission decreases proton number relative to neutron number, positron decay happens typically in large "proton-rich" radionuclides. Positron decay results in nuclear transmutation, changing an atom of one chemical element into an atom of an element with an atomic number that is less by one unit. Positron emission occurs only very rarely naturally on earth, when induced by a cosmic ray or from one in a hundred thousand decays of pot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia. Scandium is present in most of the deposits of rare-earth and uranium compounds, but it is extracted from these ores in only a few mines worldwide. Because of the low availability and difficulties in the preparation of metallic scandium, which was first done in 1937, applications for scandium were not developed until the 1970s, when the positive effects of scandium on aluminium alloys were discovered, and its use in such alloys remains its only major application. The global trade of scandium oxide is 15–20 tonnes per year. The properties of scandium compounds are intermediate between those of aluminium and yttrium. A diagonal relationship exists betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secular Equilibrium
In nuclear physics, secular equilibrium is a situation in which the quantity of a radioactive isotope remains constant because its production rate (e.g., due to decay of a parent isotope) is equal to its decay rate. In radioactive decay Secular equilibrium can occur in a radioactive decay chain only if the half-life of the daughter radionuclide B is much shorter than the half-life of the parent radionuclide A. In such a case, the decay rate of A and hence the production rate of B is approximately constant, because the half-life of A is very long compared to the time scales considered. The quantity of radionuclide B builds up until the number of B atoms decaying per unit time becomes equal to the number being produced per unit time. The quantity of radionuclide B then reaches a constant, ''equilibrium'' value. Assuming the initial concentration of radionuclide B is zero, full equilibrium usually takes several half-lives of radionuclide B to establish. The quantity of radionuclide B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOTA (chelator)
DOTA (also known as tetraxetan) is an organic compound with the formula (CH2CH2NCH2CO2H)4. The molecule consists of a central 12-membered tetraaza (i.e., containing four nitrogen atoms) ring. DOTA is used as a complexing agent, especially for lanthanide ions. Its complexes have medical applications as contrast agents and cancer treatments. Terminology The acronym DOTA (for dodecane tetraacetic acid) is shorthand for both the tetracarboxylic acid and its various conjugate bases. In the area of coordination chemistry, the tetraacid is called H4DOTA and its fully deprotonated derivative is DOTA4−. Many related ligands are referred to using the DOTA acronym, although these derivatives are generally not ''tetra''carboxylic acids or the conjugate bases. Structure DOTA is derived from the macrocycle known as cyclen. The four secondary amine groups are modified by replacement of the N-H centers with N-CH2CO2H groups. The resulting aminopolycarboxylic acid, upon ionization of the carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
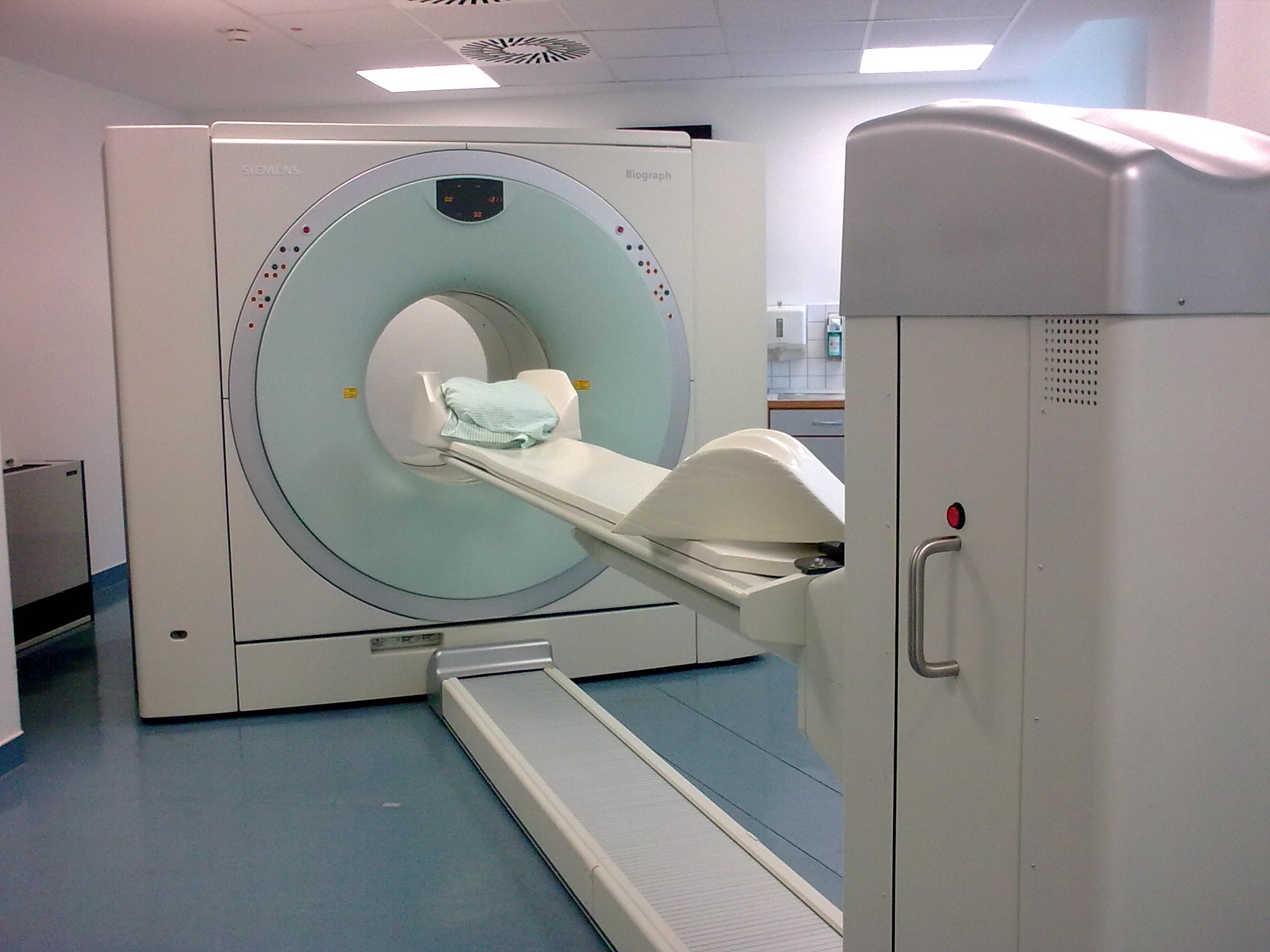
PET-CT
Positron emission tomography–computed tomography (better known as PET-CT or PET/CT) is a nuclear medicine technique which combines, in a single gantry, a positron emission tomography (PET) scanner and an x-ray computed tomography (CT) scanner, to acquire sequential images from both devices in the same session, which are combined into a single superposed ( co-registered) image. Thus, functional imaging obtained by PET, which depicts the spatial distribution of metabolic or biochemical activity in the body can be more precisely aligned or correlated with anatomic imaging obtained by CT scanning. Two- and three-dimensional image reconstruction may be rendered as a function of a common software and control system. PET-CT has revolutionized medical diagnosis in many fields, by adding precision of anatomic localization to functional imaging, which was previously lacking from pure PET imaging. For example, many diagnostic imaging procedures in oncology, surgical planning, radia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOTATATE
DOTA-TATE (DOTATATE, DOTA-octreotate, oxodotreotide, DOTA-(Tyr3)-octreotate, and DOTA-0-Tyr3-Octreotate) is an eight amino acid long peptide, with a covalently bonded DOTA bifunctional chelator. DOTA-TATE can be reacted with the radionuclides gallium-68 (T1/2 = 68 min), lutetium-177 (T1/2 = 6.65 d) and copper-64 (T1/2 = 12.7 h) to form radiopharmaceuticals for positron emission tomography (PET) imaging or radionuclide therapy. 177Lu DOTA-TATE therapy is a form of peptide receptor radionuclide therapy (PRRT) which targets somatostatin receptors (SSR). In that form of application it is a form of targeted drug delivery. Chemistry and mechanism of action DOTA-TATE is a compound containing tyrosine3-octreotate, an SSR agonist, and the bifunctional chelator DOTA (tetraxetan). SSRs are found with high density in numerous malignancies, including CNS, breast, lung, and lymphatics. The role of SSR agonists (i.e. somatostatin and its analogs such as octreotide, somatuline and va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |