|
STX10
Syntaxin-10 (STX10) is a SNARE protein that is encoded by the ''STX10'' gene. This protein is found in most vertebrates (including humans) but is noticeably absent from mice. As with other SNARE proteins, STX10 facilitates vesicle fusion and thus is important for intracellular trafficking of proteins and other cellular components. More specifically, STX10 has been implicated in endosome to Golgi trafficking of the mannose 6-phosphate receptor and glucose transporter type 4. STX10 has been detected in the trans-Golgi network (TGN) by immunofluorescence. Structure and function Human STX10 is a 249 amino acid protein that has three N-terminal α-helices and a single SNARE domain followed by a single-pass transmembrane domain. Human STX10 is 60% identical to human STX6. STX10 is structurally classified as a Qc-SNARE (contributes a glutamine (Q) residue in the formation of the assembled core SNARE complex) and is functionally classified as a t-SNARE (or target-SNARE which is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SNARE
SNARE proteins – " SNAP REceptor" – are a large protein family consisting of at least 24 members in yeasts, more than 60 members in mammalian cells, and some numbers in plants. The primary role of SNARE proteins is to mediate vesicle fusion – the fusion of vesicles with the target membrane; this notably mediates exocytosis, but can also mediate the fusion of vesicles with membrane-bound compartments (such as a lysosome). The best studied SNAREs are those that mediate the neurotransmitter release of synaptic vesicles in neurons. These neuronal SNAREs are the targets of the neurotoxins responsible for botulism and tetanus produced by certain bacteria. Types SNAREs can be divided into two categories: ''vesicle'' or ''v-SNAREs'', which are incorporated into the membranes of transport vesicles during budding, and ''target'' or ''t-SNAREs'', which are associated with nerve terminal membranes. Evidence suggests that t-SNAREs form stable subcomplexes which serve as guides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand- helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VAMP3
Vesicle-associated membrane protein 3 is a protein that in humans is encoded by the ''VAMP3'' gene. Function Synaptobrevins/VAMPs, syntaxins, and the 25-kD synaptosomal-associated protein are the main components of a protein complex involved in the docking and/or fusion of synaptic vesicles with the presynaptic membrane. This gene is a member of the vesicle-associated membrane protein (VAMP)/synaptobrevin family. Because of its high homology to other known VAMPs, its broad tissue distribution, and its subcellular localization, the protein encoded by this gene was shown to be the human equivalent of the rodent cellubrevin. In platelets the protein resides on a compartment that is not mobilized to the plasma membrane on calcium or thrombin stimulation. Interactions VAMP3 has been shown to interact with * BCAP31, * BVES, * SNAP23, * STX4 Syntaxin-4 is a protein that in humans is encoded by the ''STX4'' gene. Interactions STX4 has been shown to interact with: * Gelsolin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
STX16
Syntaxin-16 is a protein that in humans is encoded by the ''STX16'' gene. It has been associated with pseudohypoparathyroidism type Ib. Losing this gene causes loss of methylation at GNAS1 exon A/B. Interactions STX16 has been shown to interact with VAMP4 Vesicle-associated membrane protein 4 is a protein that in humans is encoded by the ''VAMP4'' gene. Function Synaptobrevins/VAMPs, syntaxins, and the 25-kD synaptosomal-associated protein SNAP25 are the main components of a protein complex in .... References Further reading * * * * * * * {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VTI1A
Vesicle transport through interaction with t-SNAREs homolog 1A is a protein that in humans is encoded by the ''VTI1A'' gene In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b .... The protein encoded by the VTI1A gene is a vesicular- SNARE (v-SNARE) protein which is located in the membranes of target vesicle compartments. References Further reading * * * * * * * * * * {{gene-10-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases, and glutamine must be obtained from the diet. It is encoded by the codons CAA and CAG. In human blood, glutamine is the most abundant free amino acid. The dietary sources of glutamine include especially the protein-rich foods like beef, chicken, fish, dairy products, eggs, vegetables like beans, beets, cabbage, spinach, carrots, parsley, vegetable juices and also in wheat, papaya, Brussels sprouts, celery, kale and fermented foods like miso. Functions Glutamine plays a role in a variety of biochemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
STX6
Syntaxin-6 is a protein that in humans is encoded by the ''STX6'' gene. Interactions STX6 has been shown to interact with SNAP23, VAMP3 and VAMP4. N terminal protein domain The protein domain Syntaxin 6 N terminal protein domain is a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) found in endosomal transport vesicles. It is part of the family, of target SNAREs (t-SNAREs). It is a vital aid to exporting and importing cell cargo through a process called cell trafficking. Its SNARE motif shows significant homology to both syntaxin 1a and S25C, indicating similarity through evolutionary conservation. The structure of the syntaxin 6 N-terminal domain shows strong structural similarity with the N-terminal domains of syntaxin 1a, Sso1p, and Vam3p; despite a very low level of sequence similarity. SNARE functions essentially as a tether to hold the vesicle. The cytoplasmic regions of SNARE found on transport vesicles and target membranes interact, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
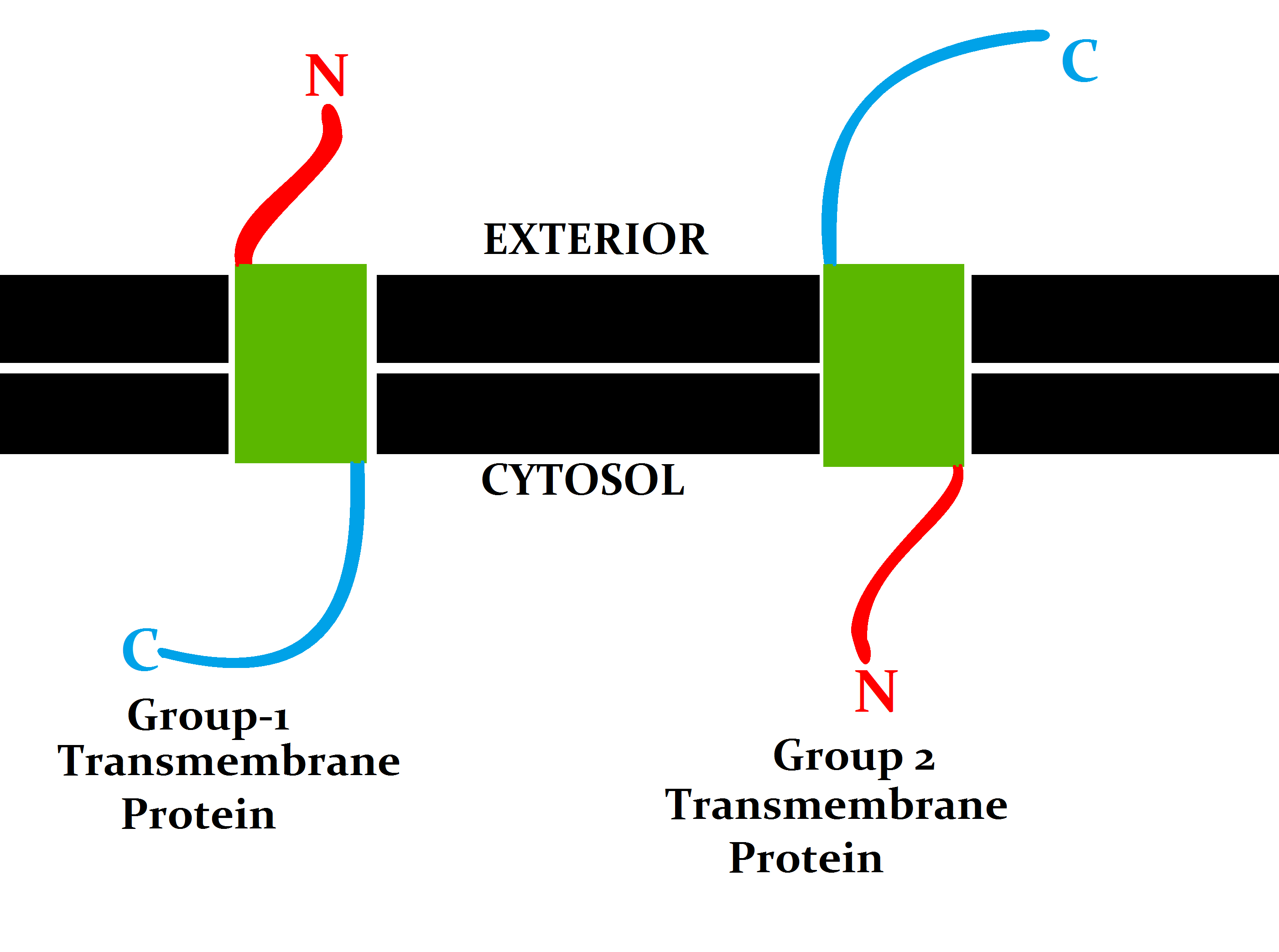
Transmembrane Protein
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer af ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |