|
STAU1
Double-stranded RNA-binding protein Staufen homolog 1 is a protein that in humans is encoded by the ''STAU1'' gene. Staufen is a member of the family of double-stranded RNA (dsRNA)-binding proteins involved in the transport and/or localization of mRNAs to different subcellular compartments and/or organelles. These proteins are characterized by the presence of multiple dsRNA-binding domains which are required to bind RNAs having double-stranded secondary structures. The human homologue of staufen encoded by STAU, in addition contains a microtubule-binding domain similar to that of microtubule-associated protein 1B, and binds tubulin. The STAU gene product has been shown to be present in the cytoplasm in association with the rough endoplasmic reticulum (RER), implicating this protein in the transport of mRNA via the microtubule network to the RER, the site of translation. Five transcript variants resulting from alternative splicing of STAU gene and encoding three isoforms have b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
STAU1 DsRBD4 Details Of Interactions With SARF SBS RNA
Double-stranded RNA-binding protein Staufen homolog 1 is a protein that in humans is encoded by the ''STAU1'' gene. Staufen is a member of the family of double-stranded RNA (dsRNA)-binding proteins involved in the transport and/or localization of mRNAs to different subcellular compartments and/or organelles. These proteins are characterized by the presence of multiple dsRNA-binding domains which are required to bind RNAs having double-stranded secondary structures. The human homologue of staufen encoded by STAU, in addition contains a microtubule-binding domain similar to that of microtubule-associated protein 1B, and binds tubulin. The STAU gene product has been shown to be present in the cytoplasm in association with the rough endoplasmic reticulum (RER), implicating this protein in the transport of mRNA via the microtubule network to the RER, the site of translation. Five transcript variants resulting from alternative splicing of STAU gene and encoding three isoforms have b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staufen (protein)
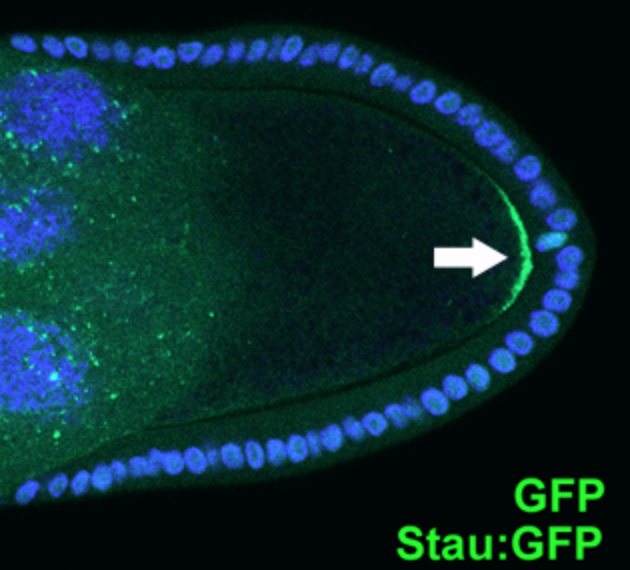
Staufen is a protein product of a maternally expressed gene first identified in ''Drosophila melanogaster.'' The protein has been implicated in helping regulate genes important in determination of gradients that set up the anterior posterior axis such as bicoid and oskar. Staufen proteins, abbreviated Stau, are necessary for cell localization during the oogenesis and zygotic development. It is involved in targeting of the messenger RNA encoding these genes to the correct pole of the egg cell. Human homologs of this protein include STAU1 and STAU2. Forms Staufen proteins are categorized under a family of double stranded RNA-binding proteins. Many homologs of Staufen proteins exist depending on the organism. The mammalian homologs of Staufen include STAU1 and STAU2. The gene encoding the STAU1 protein is found along the long arm of chromosome 20, while the gene encoding STAU2 is found on chromosome 8. These proteins are identified by the presence of double-stranded RNA binding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress Granule
Stress granules are dense aggregations in the cytosol composed of proteins and RNAs that appear when the cell is under stress. The RNA molecules stored are stalled translation pre-initiation complexes: failed attempts to make protein from mRNA. Stress granules are 100–200 nm in size (when biochemically purified), not surrounded by membrane, and associated with the endoplasmatic reticulum. Note that there are also nuclear stress granules. This article is about the cytosolic variety. Proposed functions The function of stress granules remains largely unknown. Stress granules have long been proposed to have a function to protect RNAs from harmful conditions, thus their appearance under stress. The accumulation of RNAs into dense globules could keep them from reacting with harmful chemicals and safeguard the information coded in their RNA sequence. Stress granules might also function as a decision point for untranslated mRNAs. Molecules can go down one of three paths: further ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tumor Suppressor Gene
A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results in a loss or reduction in its function. In combination with other genetic mutations, this could allow the cell to grow abnormally. The loss of function for these genes may be even more significant in the development of human cancers, compared to the activation of oncogenes. TSGs can be grouped into the following categories: caretaker genes, gatekeeper genes, and more recently landscaper genes. Caretaker genes ensure stability of the genome via DNA repair and subsequently when mutated allow mutations to accumulate. Meanwhile, gatekeeper genes directly regulate cell growth by either inhibiting cell cycle progression or inducing apoptosis. Lastly landscaper genes regulate growth by contributing to the surrounding environment, when mutated can cause an envir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARF1
ADP-ribosylation factor 1 is a protein that in humans is encoded by the ''ARF1'' gene. Function ADP-ribosylation factor 1 (ARF1) is a member of the human ARF gene family. The family members encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking as activators of phospholipase D. The gene products, including 6 ARF proteins and 11 ARF-like proteins, constitute a family of the RAS superfamily. The ARF proteins are categorized as class I (ARF1, ARF2 and ARF3), class II (ARF4 and ARF5) and class III (ARF6), and members of each class share a common gene organization. The ARF1 protein is localized to the Golgi apparatus and has a central role in intra-Golgi transport. Multiple alternatively spliced transcript variants encoding the same protein have been found for this gene. The major mechanism of action of Brefeldin A is through inhibition of ARF1. Interactions ARF1 has been shown ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UPF1
Regulator of nonsense transcripts 1 is a protein that in humans is encoded by the ''UPF1'' gene. Function This gene encodes a protein that is part of a post-splicing multiprotein complex, the exon junction complex, involved in both mRNA nuclear export and mRNA surveillance. mRNA surveillance detects exported mRNAs with truncated open reading frames and initiates nonsense-mediated mRNA decay (NMD). When translation ends upstream from the last exon-exon junction, this triggers NMD to degrade mRNAs containing premature stop codons. This protein is located in both the cytoplasm and nucleus of the cell. When translation ends, it interacts with the protein that is a functional homolog of yeast Upf2p to trigger mRNA decapping. Use of multiple polyadenylation sites has been noted for this gene. Interactions UPF1 has been shown to interact with: * DCP1A, * DCP2, * SMG1, * UPF2, * UPF3A, and * UPF3B Regulator of nonsense transcripts 3B is a protein that in humans is encoded by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinesin
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP) (thus kinesins are ATPases, a type of enzyme). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport. Discovery Kinesins were discovered in 1985, based on their motility in cytoplasm extruded from the giant axon of the squid. They turned out as MT-based anterograde intracellular trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynein
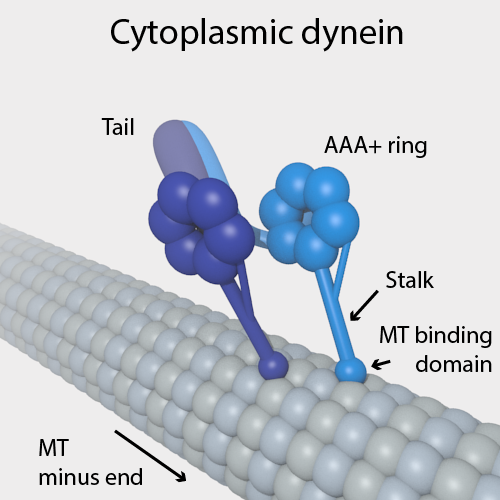
Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport. Classification Dyneins can be divided into two groups: cytoplasmic dyneins and axonemal dyneins, which are also called ciliary or flagellar dyneins. * cytoplasmic ** heavy chain: DYNC1H1, DYNC2H1 ** intermediate chain: DYNC1I1, DYNC1I2 ** light intermediate chain: DYNC1LI1, DYNC1LI2, DYNC2LI1 ** light chain: DYNLL1, DYNLL2, DYNLRB1, DYNLRB2, DYNLT1, DYNLT3 * axo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double-stranded RNA-binding Protein Staufen Homolog 1
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA and RNA. Dictated by specific hydrogen bonding patterns, "Watson–Crick" (or "Watson–Crick–Franklin") base pairs (guanine–cytosine and adenine–thymine) allow the DNA helix to maintain a regular helical structure that is subtly dependent on its nucleotide sequence. The complementary nature of this based-paired structure provides a redundant copy of the genetic information encoded within each strand of DNA. The regular structure and data redundancy provided by the DNA double helix make DNA well suited to the storage of genetic information, while base-pairing between DNA and incoming nucleotides provides the mechanism through which DNA polymerase replicates DNA and RNA polymerase transcribes DNA into RNA. Many DNA-binding proteins c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alternative Splicing
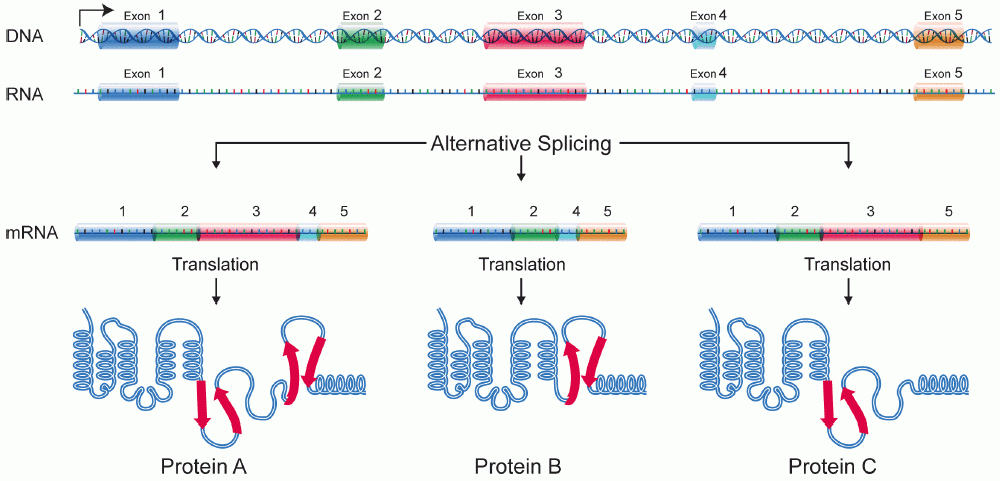
Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be included within or excluded from the final, processed messenger RNA (mRNA) produced from that gene. This means the exons are joined in different combinations, leading to different (alternative) mRNA strands. Consequently, the proteins translated from alternatively spliced mRNAs will contain differences in their amino acid sequence and, often, in their biological functions (see Figure). Biologically relevant alternative splicing occurs as a normal phenomenon in eukaryotes, where it increases the number of proteins that can be encoded by the genome. In humans, it is widely believed that ~95% of multi-exonic genes are alternatively spliced to produce functional alternative products from the same gene but many scientists believe that most o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rough Endoplasmic Reticulum
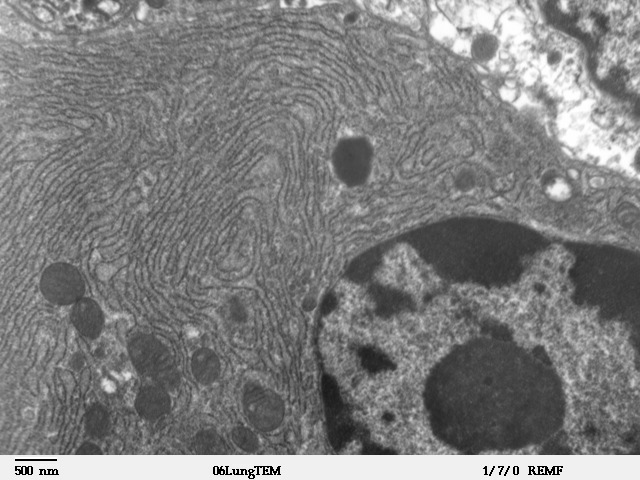
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |