|
S-Adenosylmethioninamine
''S''-Adenosylmethioninamine is a substrate that is required for the biosynthesis of polyamines including spermidine, spermine, and thermospermine. It is produced by decarboxylation of ''S''-adenosyl methionine. See also * Adenosylmethionine decarboxylase * Spermidine synthase * Spermine synthase Spermine synthase (, ''spermidine aminopropyltransferase'', ''spermine synthetase'') is an enzyme that converts spermidine into spermine. This enzyme catalyses the following chemical reaction : S-adenosylmethioninamine + spermidine \rightlefthar ... * Thermospermine synthase (ACAULIS5) References Nucleosides Purines Organosulfur compounds Cations {{biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosylmethionine Decarboxylase
The enzyme adenosylmethionine decarboxylase () catalyzes the conversion of ''S''-adenosyl methionine to ''S''-adenosylmethioninamine. Polyamines such as spermidine and spermine are essential for cellular growth under most conditions, being implicated in many cellular processes including DNA, RNA and protein synthesis. S-adenosylmethionine decarboxylase (AdoMetDC) plays an essential regulatory role in the polyamine biosynthetic pathway by generating the n-propylamine residue required for the synthesis of spermidine and spermine from putrescein. Unlike many amino acid decarboxylases AdoMetDC uses a covalently bound pyruvate residue as a cofactor rather than the more common pyridoxal 5'-phosphate. These proteins can be divided into two main groups which show little sequence similarity either to each other, or to other pyruvoyl-dependent amino acid decarboxylases: class I enzymes found in bacteria and archaea, and class II enzymes found in eukaryotes. In both groups the active enzy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
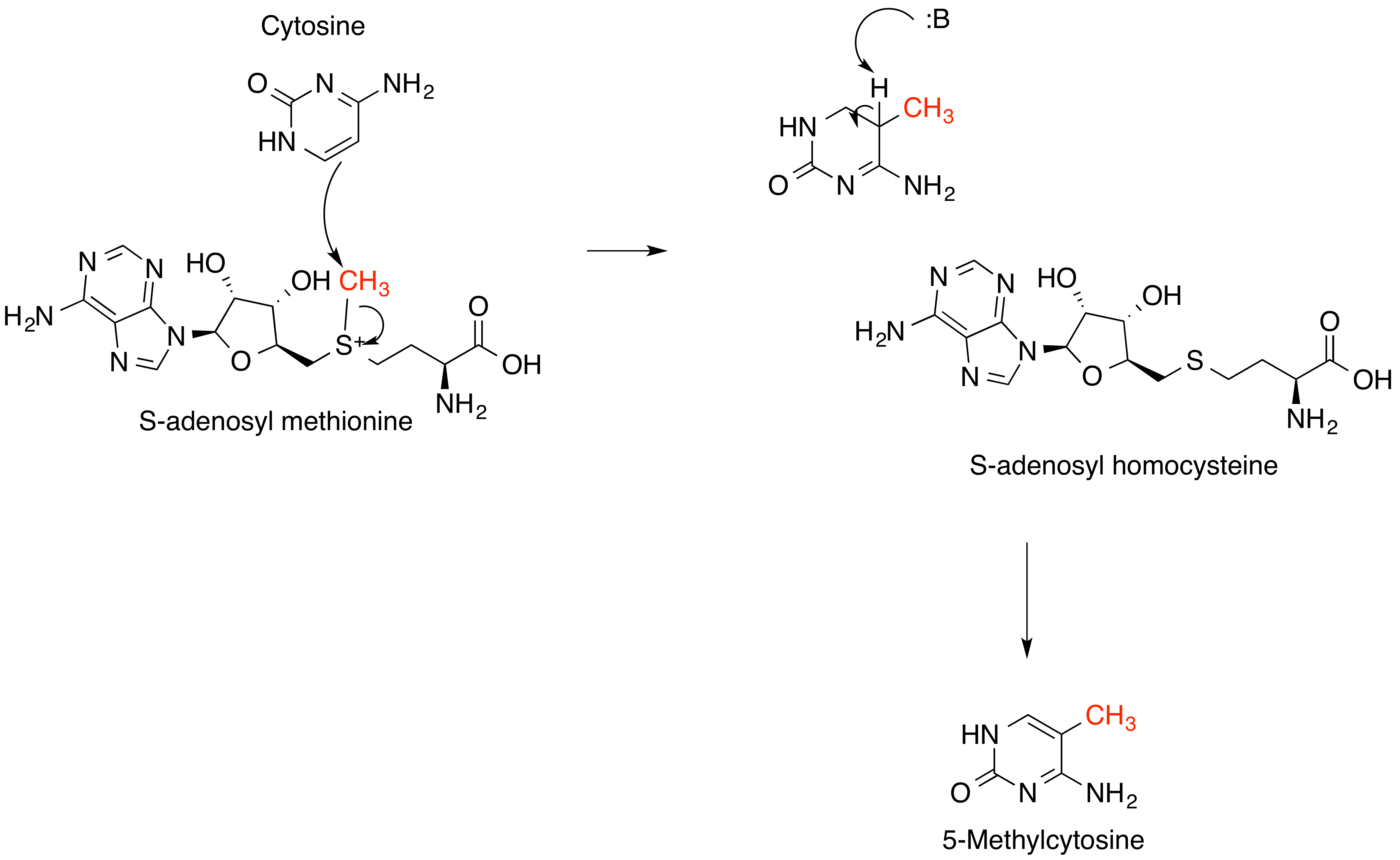
S-Adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermidine Synthase
Spermidine synthase is an enzyme () that catalyzes the transfer of the propylamine group from ''S''-adenosylmethioninamine to putrescine in the biosynthesis of spermidine. The systematic name is S-adenosyl 3-(methylthio)propylamine:putrescine 3-aminopropyltransferase and it belongs to the group of aminopropyl transferases. It does not need any cofactors. Most spermidine synthases exist in solution as dimers. Specificity With exception of the spermidine synthases from ''Thermotoga maritimum'' and from ''Escherichia coli'', which accept different kinds of polyamines, all enzymes are highly specific for putrescine. No known spermidine synthase can use ''S''-adenosyl methionine. This is prevented by a conserved aspartatyl residue in the active site, which is thought to repel the carboxyl moiety of ''S''-adenosyl methionine. The putrescine-N-methyl transferase whose substrates are putrescine and ''S''-adenosyl methionine and which is evolutionary related to the spermidine synthase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate (biochemistry)
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and microscop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyamines
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature. Natural polyamines Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC). Polyamines are found in high concentrations in the mammalian brain. File:Spermidine-2D-skeletal.svg, spermidine File:Spermine.svg, spermine Synthetic polyamines Several synthetic polyamines are used in chemical industry and the research laboratory. They are mainly of interest as additives to motor oil and as co-rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermidine
Spermidine is a polyamine compound () found in ribosomes and living tissues and having various metabolic functions within organisms. It was originally isolated from semen. Function Spermidine is an aliphatic polyamine. Spermidine synthase (SPDS) catalyzes its formation from putrescine. It is a precursor to other polyamines, such as spermine and its structural isomer thermospermine. Spermidine synchronizes an array of biological processes, (such as Ca2+, Na+, K+ -ATPase) thus maintaining membrane potential and controlling intracellular pH and volume. Spermidine regulates biological processes, such as Ca2+ influx by glutamatergic N-methyl-d-aspartate receptor (NMDA receptor), which has been associated with nitric oxide synthase (NOS) and cGMP/PKG pathway activation and a decrease of Na+,K+-ATPase activity in cerebral cortex synaptosomes. Spermidine is a longevity agent in mammals due to various mechanisms of action, which are just beginning to be understood. Autophagy is the main m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermine
Spermine is a polyamine involved in cellular metabolism that is found in all Eukaryote, eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some Bacterium, bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. Antonie van Leeuwenhoek first described crystals of spermine phosphate in human semen in 1678. The name ''spermin'' was first used by the German chemists Albert Ladenburg, Ladenburg and Abel in 1888, and the correct structure of spermine was not finally established until 1926, simultaneously in England (by Dudley, Rosenheim, and Starling) and Germany (by Wrede et al.). Spermine is the chemical primarily responsible for the characteristic odor of semen. Derivative A derivative (chemistry), derivative of spermine, N1, N12-bis(ethyl)spermine (also known as BESm) was investig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermospermine
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature. Natural polyamines Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC). Polyamines are found in high concentrations in the mammalian brain. File:Spermidine-2D-skeletal.svg, spermidine File:Spermine.svg, spermine Synthetic polyamines Several synthetic polyamines are used in chemical industry and the research laboratory. They are mainly of interest as additives to motor oil and as co-r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annals Of Botany
''Annals of Botany'' is a monthly peer-reviewed scientific journal publishing experimental, theoretical and applied papers on all aspects of plant biology. The current (2022) Chief Editor is Rowan Sage, replacing John Seymour (Pat) Heslop-Harrison (University of Leicester, UK and the South China Botanical Garden appointed in 2008). The journal is owned and managed by thAnnals of Botany Company a non-profit educational charity registered with the Charity Commission for England and Wales. It is published monthly through Oxford University Press in paper form and online, and is paid for primarily by institutional annual subscriptions. Regular extra issues, published free-of-charge, focus on topical themes. The journal does not levy page charges but authors may choose to pay a standard fee to secure open access status for their papers. According to ''Journal Citation Reports'', in 2019 (published 2020) ''Annals of Botany''’s impact factor was 4.005 and was ranked 27th out of 234 jou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermine Synthase
Spermine synthase (, ''spermidine aminopropyltransferase'', ''spermine synthetase'') is an enzyme that converts spermidine into spermine. This enzyme catalyses the following chemical reaction : S-adenosylmethioninamine + spermidine \rightleftharpoons S-methyl-5'-thioadenosine + spermine Spermine synthase is an enzyme involved in polyamine biosynthesis. It is present in all eukaryotes and plays a role in a variety of biological functions in plants Its structure consists of two identical monomers of 41 kDa with three domains each, creating a homodimer formed via dimerization A dimer () (''wikt:di-, di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, Covalent bond, covalent or Intermolecular force, intermolecular. Dimers also have significant im .... The interactions between one of the three domains, the N-terminals of the monomers, is responsible for dimerization as that is where the active site is located; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermospermine Synthase
Thermospermine synthase (, ''TSPMS'', ''ACL5'' (''ACAULIS5''), ''SAC51'') is an enzyme with systematic name ''S-adenosylmethioninamine:spermidine 3-aminopropyltransferase (thermospermine synthesizing)''. This enzyme catalyses the following chemical reaction : S-adenosylmethioninamine + spermidine \rightleftharpoons S-methyl-5'-thioadenosine + thermospermine + H+ This enzyme is required for correct xylem Xylem is one of the two types of transport tissue in vascular plants, the other being phloem. The basic function of xylem is to transport water from roots to stems and leaves, but it also transports nutrients. The word ''xylem'' is derived from ... specification through regulation of the lifetime of the xylem elements. References External links * {{Portal bar, Biology, border=no EC 2.5.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |